Back to article: Could de-stressing the brain be the solution for long-term weight loss?
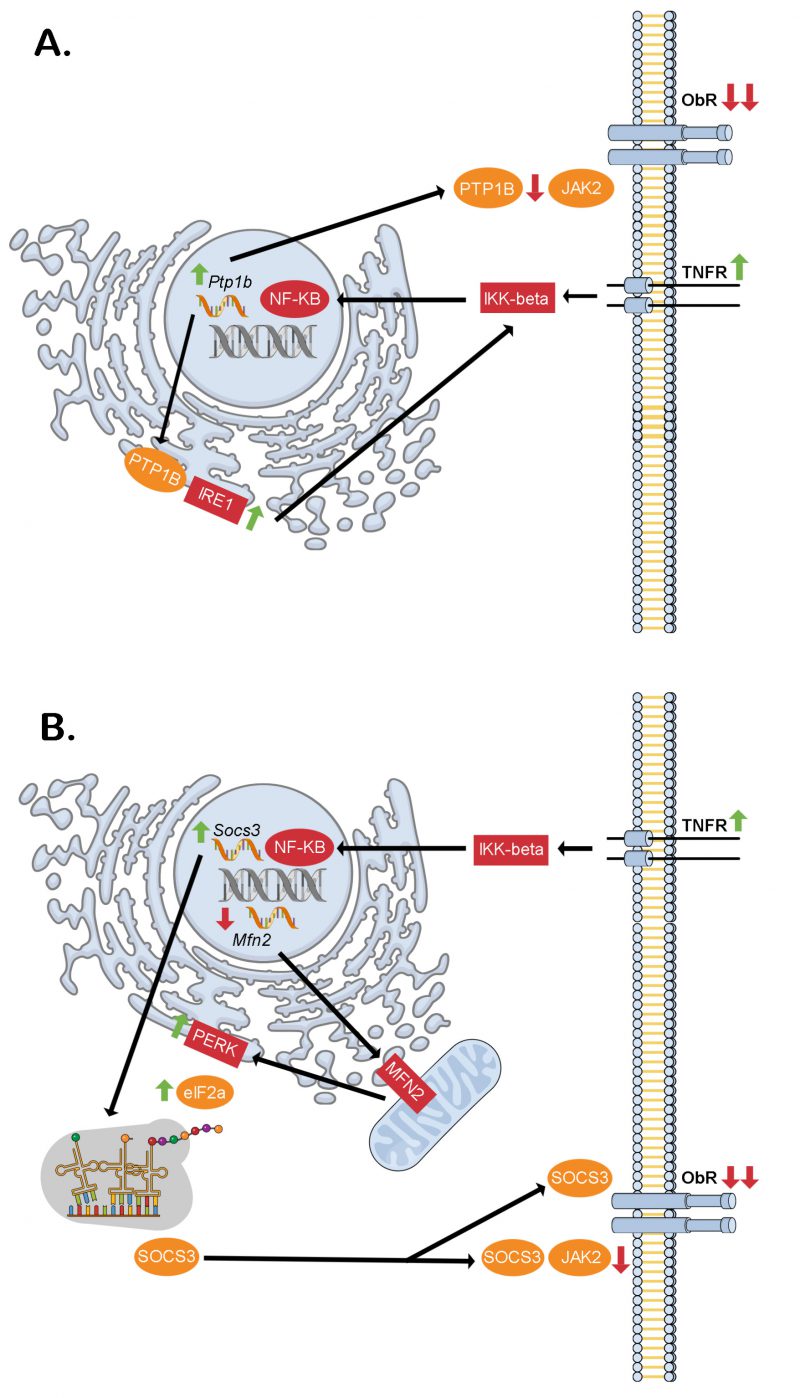
FIGURE 2: Proposed intracellular signaling cascades liking inflammation, ER stress and hypothalamic neuronal leptin resistance in obesity. (A) Through the dual phosphatase action of PTP1B at the ER (stimulatory on IRE1) and cell membrane (inhibitory on JAK2) downstream of TNF-α receptor activation, hypothalamic neuronal leptin receptor signaling may be blunted contributing to increased food intake and obesity. (B) Similarly, through the dual action of NF-KB of increasing Socs3 transcription and decreasing Mfn2 transcription, hypothalamic neuronal leptin receptor signaling may be blunted contributing to increased food intake and obesity. This would be through decreased mitochondrial MFN2 leading to reduced ER-mitochondrial contacts thereby causing ER stress. The PERK-eIF2α arm of this response mediates alternative translation of Socs3 mRNA of a more stable SOCS3 variant, which lacks an amino terminus tail containing a lysine residue that is normally ubiquitinated sending the full-length SOCS3 to the proteasome for degradation. eIF2a – elongation initiation factor 2 alpha, IKK-beta – inhibitor of kappa beta kinase beta, IRE1 – inositol requiring enzyme 1, JAK2 – janus kinase 2, Mfn2 – Mitofusin-2, NK-κB – necrosis factor kappa beta, ObR – leptin receptor, PERK – protein kinase R (PKR)-like ER kinase, PTP1B – protein tyrosine phosphatase 1B, Socs3 – suppressor of cytokine signaling 3, TNFR – tumor necrosis factor receptor.