Back to article: Development of novel methods that monitor necroptosis and the release of DAMPs at the single cell resolution
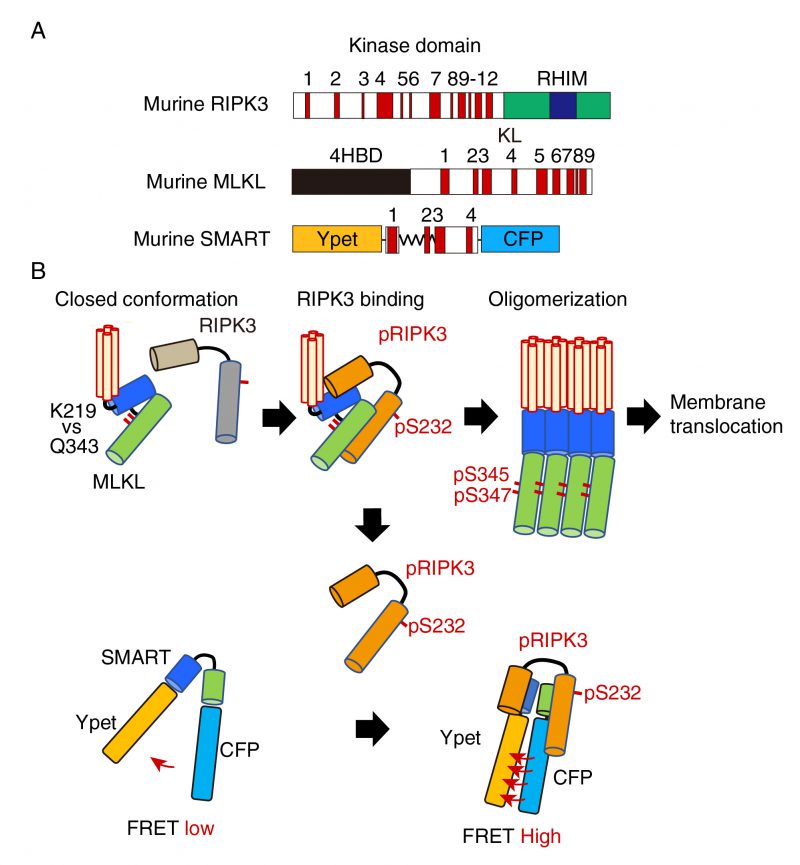
FIGURE 1: A model for the mechanism how SMART senses necroptosis. (A) Domain structures of murine RIPK3, MLKL, and diagram of SMART. mRIPK3 is composed of N-terminal kinase domain that contains twelve helices and C-terminal RIP homotypic interaction motif (RHIM) domain. mMLKL is composed of N-terminal four helices bundle domain (4HBD) and C-terminal kinase-like (KL) domain. mSMART is composed of N-terminal Ypet and C-terminal CFP where a modified fragment containing α 1 to 4 helices of MLKL is inserted in the intervening region. Numbers indicate each α helix. (B) In unstimulated cells, endogenous MLKL adopts an inactive conformation via hydrogen bond between K219 and Q343, thereby suppressing spontaneous oligomer formation. Following necroptosis induction, activated RIPK3 (pRIPK3) binds to MLKL, resulting in phosphorylation of MLKL that induces conformational changes and subsequent oligomer formation. Oligomerized MLKL translocates to the plasma membrane and induces membrane rupture. Since SMART does not contain K216, SMART appears to adopt an open conformation. Weak FRET signals of SMART are detected in unstimulated cells. Once the oligomerized MLKL is released from activated RIPK3 and translocates to the plasma membrane, activated RIPK3 subsequently binds to induces conformational changes of SMART, increasing the FRET/CFP ratio. Thus, an increase in the FRET/CFP ratio of SMART is tightly correlated with plasma membrane translocation of oligomerized MLKL.