Back to article: Mitochondria organize the cellular proteostatic response and promote cellular senescence
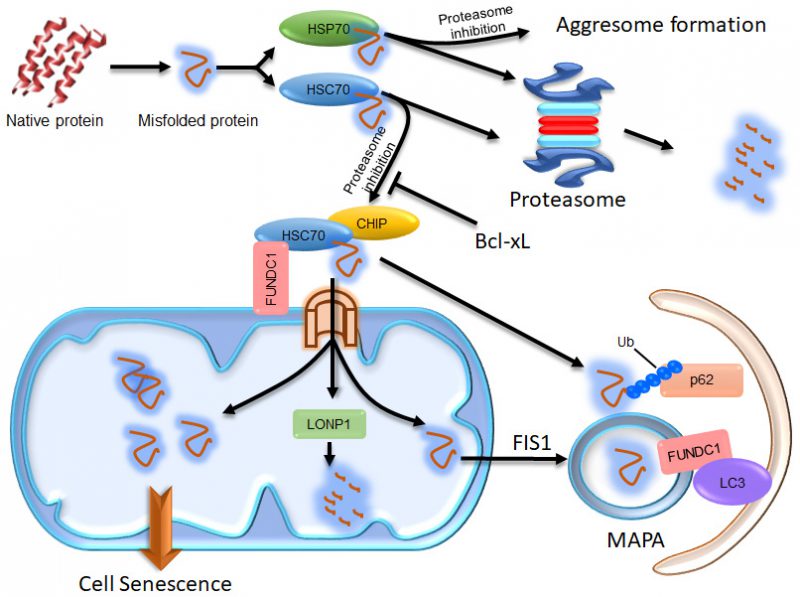
FIGURE 1: Illustration of the compensatory degradation of proteasomal substrates. The cytosolic chaperones HSP70 and HSC70 take their clients to the proteasome for degradation. If proteasomal activity is inhibited, HSP70 proteins take their clients to the microtubule organization center to form aggresomes, which may facilitate the autophagic degradation of the client proteins. HSC70 proteins, however, take their clients to mitochondria through the interaction of HSC70 with FUNDC1. Some of the client proteins are then imported into the mitochondrial matrix via the TOM/TIM complex and degraded by LONP1. The remains of the client proteins imported into the mitochondrial matrix may concentrate in certain region and become segregated from the mitochondrial network in a FIS-dependent manner to form MAPAs. The HSC70 clients at the surface of mitochondria can be ubiquitinated by the E3 ligase CHIP, which endows them with the ability to recruit p62 proteins. The ubiquitinated proteins and p62 at the surface of mitochondria and some of the mitochondrial membrane proteins, including FUNDC1, are all incorporated into the MAPAs. FUNDC1 can mediate recognition of the MAPAs by autophagosomes via its interaction with LC3. If the proteasomal client proteins imported into the mitochondrial matrix are not cleared in time, excessive accumulation of these proteins may disturb the mitochondrial function, consequently resulting in cellular senescence.