Editorial:
Cell Stress, Vol. 7, No. 7, pp. 50 - 58; doi: 10.15698/cst2023.07.281
Molecular targets of spermidine: implications for cancer suppression
1 Institute of Molecular Biosciences, NAWI Graz, University of Graz, Graz, Austria.
2 Field of Excellence BioHealth, University of Graz, Graz, Austria.
3 BioTechMed Graz, Graz, Austria.
Keywords: spermidine, cancer, molecular targets, immunosurveillance, hypusination, autophagy.
Abbreviatons:
DFMO – difluoromethylornithine;
eIF5A – eukaryotic translation initiation factor 5A;
FAO – fatty acid oxidation;
MTP – mitochondrial trifunctional protein;
NAFLD – non-alcoholic fatty liver disease;
NASH – non-alcoholic steatohepatitis;
NMDAR – neuronal glutamate-activated N-methyl-D-aspartate re-ceptor;
ODC – ornithine decarboxylase;
ROS – reactive oxygen species;
TAZ – transcriptional coactivator with PDZ-binding motif;
YAP – Yes-associated protein.
Received originally: 15/05/2023 Received in revised form: 14/06/2023
Accepted: 21/06/2023
Published: 10/07/2023
Correspondence:
Frank Madeo, Institute of Molecular Biosciences, NAWI Graz, University of Graz, Graz, Austria; frank.madeo@uni-graz.at
Conflict of interest statement: F.M. is a scientific cofounder of Samsara Therapeutics, a company that develops novel pharmacological autophagy inducers. F.M. has equity interests in and is advisor of The Longevity Labs (TLL). S.J.H. and A.Z. declare no conflict of interest.
Please cite this article as: Andreas Zimmermann, Sebastian J. Hofer and Frank Madeo (2023). Molecular targets of spermidine: implications for cancer suppression. Cell Stress 7(7): 50-58. doi: 10.15698/cst2023.07.281
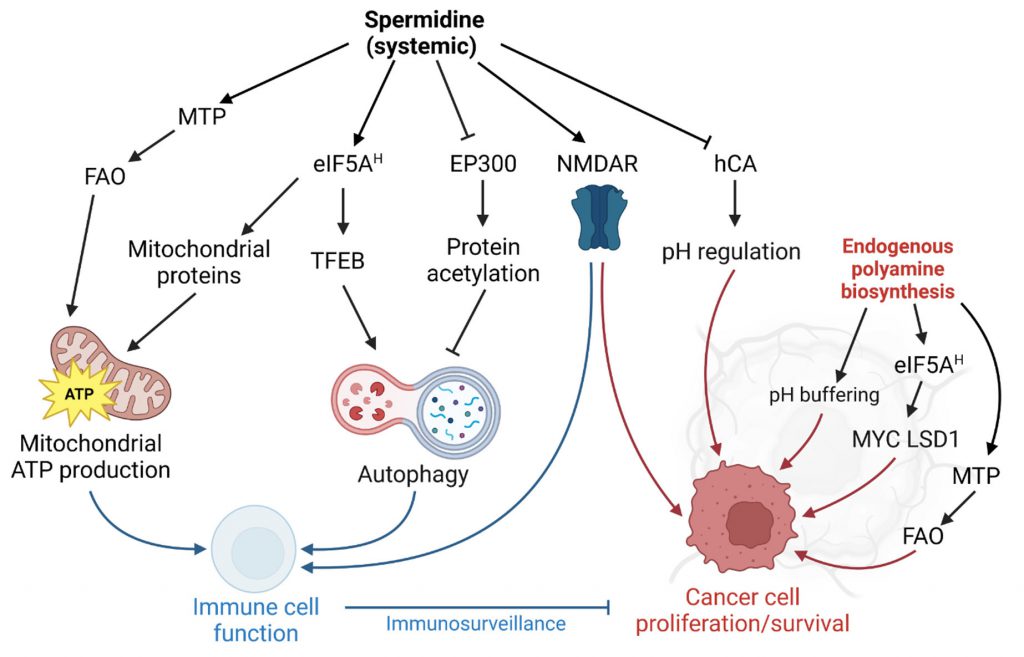
Spermidine is a ubiquitous, natural polyamine with geroprotective features. Supplementation of spermidine extends the lifespan of yeast, worms, flies, and mice, and dietary spermidine intake correlates with reduced human mortality. However, the crucial role of polyamines in cell proliferation has also implicated polyamine metabolism in neoplastic diseases, such as cancer. While depleting intracellular polyamine biosynthesis halts tumor growth in mouse models, lifelong external spermidine administration in mice does not increase cancer incidence. In contrast, a series of recent findings points to anti-neoplastic properties of spermidine administration in the context of immunotherapy. Various molecular mechanisms for the anti-aging and anti-cancer properties have been proposed, including the promotion of autophagy, enhanced translational control, and augmented mitochondrial function. For instance, spermidine allosterically activates mitochondrial trifunctional protein (MTP), a bipartite protein complex that mediates three of the four steps of mitochondrial fatty acid b-oxidation. Through this action, spermidine supplementation is able to restore MTP-mediated mitochondrial respiratory capacity in naïve CD8+ T cells to juvenile levels and thereby improves T cell activation in aged mice. Here, we put this finding into the context of the previously described molecular target space of spermidine.
INTRODUCTION
Polyamines are polycationic small molecules produced endogenously via the decarboxylation of the non-proteinogenic amino acid ornithine by ornithine decarboxylase (ODC). The product of this reaction, putrescine, is converted to spermidine and spermine by the stepwise addition of propylamine groups via spermidine (SRM) and spermine synthases (SMS), respectively. In addition, cellular polyamine pools are regulated by dietary uptake, microbial production and degradation [1]. Polyamine levels are homeostatically controlled by tight regulatory networks that quickly fine-tune endogenous production to the cellular supply.
–
Despite this stringent regulation, multiple reports, including a recent study by Al-Habsi et al. [2], show that polyamine levels, especially spermidine, decline significantly in different cell types and tissues of aging organisms [1]. While the molecular etiology of this phenomenon remains elusive, spermidine supplementation has emerged as a promising strategy to revert aging-associated cellular and organismal dysfunction [3][4][5]. Spermidine promotes several cellular processes that are linked to longevity, e.g., (i) autophagy, (ii) protein synthesis efficiency, and (iii) mitochondrial function. The latter results in increased oxidative phosphorylation rates and improved adenosine triphosphate (ATP) production. Although these processes are intertwined through several mechanistic nodes (e.g., mitochondrial oxidative phosphorylation depends on autophagy as well as spermidine-mediated translation efficiency) [4][6], a universal molecular target that integrates the heterogenous cellular effects of spermidine has not been identified and might well be cell type- and tissue-specific. We have recently summarized the complexity of molecular interactors targeted by spermidine in the context of aging and autophagy [7]. While the exact hierarchical clustering of specific and relevant molecular factors for the lifespan-extending properties of spermidine supplementation remains vague, the activation of eukaryotic translation initiation factor 5A (eIF5A) via the posttranslational modification hypusination, plays an important role in the spermidine-mediated improvement of autophagy and mitochondrial function, building the basis of its geroprotective properties [7].
–
Despite the beneficial effects of spermidine supplementation on healthspan and longevity, polyamines have also been implicated in cancer cell growth [8]. ODC, the rate-limiting enzyme of polyamine anabolism, is transcriptionally activated by the oncogene MYC, suggesting that cellular transformation involves an upregulation of polyamines. Indeed, polyamine metabolism is often dysregulated in cancer cells [9], which can amount to a polyamine “addiction” of certain cancers, such as pancreatic ductal adenocarcinoma [8]. Consequently, some therapeutic approaches targeting the transport, anabolism or catabolism of polyamines, most notably the specific inhibition of ODC with the inhibitor difluoromethylornithine (DFMO), have been explored to treat neoplastic diseases [8][10].
–
The notion that spermidine and polyamines in general are essential for cancer cell proliferation has been regarded as a major caveat in dietary supplementation regimes. However, epidemiological studies challenge this paradigm as they indicate a negative correlation of dietary spermidine intake and cancer-related mortality [11][12]. Despite decades of research, pharmacological inhibition of polyamine production did not lead to cancer therapies for patients so far [10][13]. Conversely, growing body of evidence suggests that spermidine has important cell-autonomous and non-autonomous cancer-preventive functions, especially through enhanced anticancer immunosurveillance, i.e., the efficient removal of malignant cells by the immune system [14]. The Janus-faced role of spermidine in cancer vs. other cells warrants a closer look at the molecular targets and their respective role in normal and transformed cells and tissues.
THE MOLECULAR TARGET SPACE OF SPERMIDINE
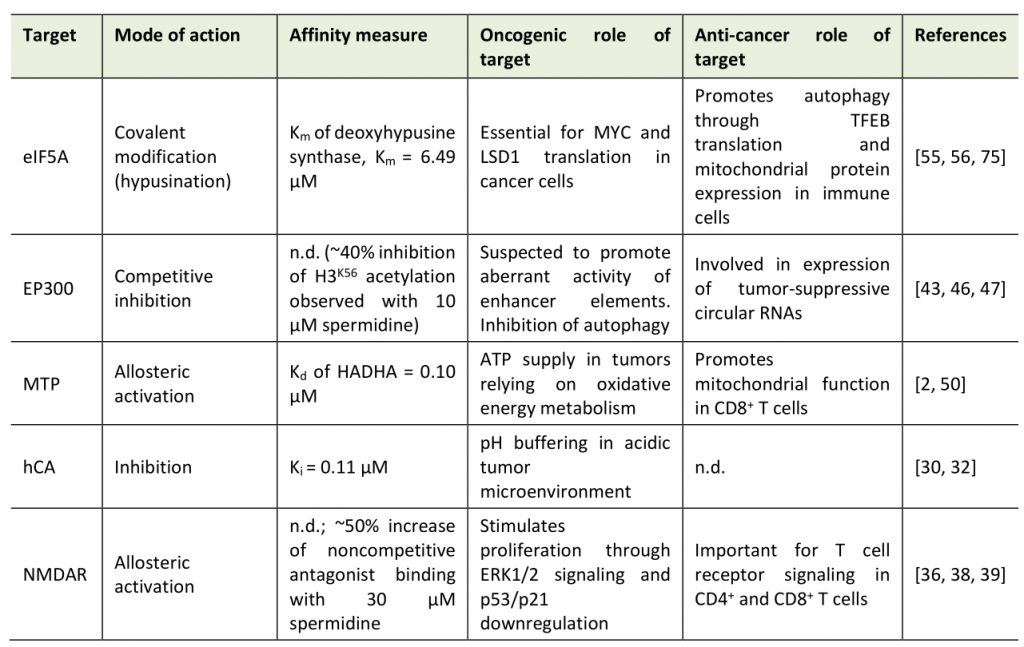
The molecular target space of spermidine can be categorized into three classes: (1) non-specific interactions; (2) site-specific, orthosteric and allosteric interactions; and (3) covalent binding (Table 1).
–
Table 1. Direct molecular targets of spermidine. |
 |
–
Non-specific interactions of spermidine
Due to its positively charged amine groups, spermidine interacts in a counterion-like, non-selective fashion with negatively charged biomolecules, which might represent a primordial function of polyamines. Indeed, early studies found spermidine bound to phosphate-containing molecules such (e.g., DNA, RNA, phospholipids, ATP) [15] with dissociation constants (Kd) between spermidine and DNA (Kd=0.49 µM) similar to those of the typical DNA-associated ion Mg2+ (Kd=0.65 µM) [16]. Although the biophysical basis for these interactions is well understood [17][18], the biological consequences are not, possibly owing to the fact that it is difficult to pinpoint the effects of spermidine treatment to such, rather unspecific, molecular interactions. Still, there is increasing evidence that spermidine has a regulatory function through non-specific interactions, for instance, via the induction of structural changes in RNA or tRNA anticodon modification [19][20]. One proposed role of spermidine is the protection of DNA against mutagens, e.g., singlet oxygen, especially during DNA replication [21]. In support of this hypothesis, yeast cells treated with spermidine are protected from different DNA-damaging noxa, like UV light and ethyl methanesulfonate [22]. In general, polyamines are effective reactive oxygen species (ROS) scavengers [23][24], which protects healthy cells on the one hand, but may also impede ROS-mediated regulated cell death in cancer cells.
–
Sequence-specific interaction of spermidine with DNA can enhance binding efficiencies of transcription factors in vitro, e.g. nuclear factor κB (NF-κB) and estrogen receptor α (ERα), which are both implicated in breast cancer [25][26]. Experiments with uranyl photo-probing demonstrated that polyamines preferentially interact with bent adenine stretches, which are also found close to NF-κB binding sites [27]. However, it is not understood whether these interactions are specifically required in cancer cells to enable proliferative transcription programs. Importantly, spermidine can also inhibit the interaction of transcription factors with DNA, at least in vitro, e.g., in the case of the malignant stem cell factor Oct-1 (encoded by the gene POU2F1) [25], and thereby potentially exerts tumor-suppressive activity in this specific setting [28].
–
Site-specific interactions of spermidine
Importantly, the alkyl chains of spermidine (and other polyamines) also mediate structural interactions (e.g., hydrogen bonds and hydrophobic interactions) in addition to the ionic, electrostatic forces [17][29]. Hence, spermidine binds other cell components, such as proteins, in a specific fashion (beyond the apparent interaction with enzymes in polyamine metabolism). For instance, spermidine inhibits human carbonic anhydrase (hCA; inhibitor constant, Ki=0.11 µM) [30] through hydrogen bond interactions with the protein and the coordinated Zn(II) ligand [31]. Carbonic anhydrases, especially isoform IX, contribute to pH regulation and the resistance to cancer cell-borne local acidosis in the tumor microenvironment [32]. However, a therapeutic use of spermidine against carbonic anhydrases might be problematic in certain scenarios: in glioblastoma, for example, tumor-associated myeloid cells (but not infiltrating CD8+ T cells) upregulate polyamine synthesis to buffer low intracellular pH in the acidic microenvironment. DFMO feeding in tumor-engrafted mice perturbs the immunosuppressive tumor microenvironment and ultimately improves survival [33].
–
Spermidine is also an allosteric activator of membrane proteins, such as neuronal glutamate-activated N-methyl-D-aspartate receptors (NMDAR), critical for memory formation [34][35][36]. Inferred from experiments with spermine, spermidine likely binds to a subunit-subunit interface between the GluN1 and GluN2B subunits of the NMDAR and thereby stabilizes the dimer in an active conformation [37]. We have previously shown that dietary spermidine supplementation improves spatial memory in aged mice, which, however, does involve signatures of increased hippocampal NMDAR signaling [6]. Interestingly, NMDARs are expressed in different cancer cell types, even in cells of non-neuronal origin, and are suspected to activate the mTOR pathway though autocrine and paracrine signaling [38]. NMDAR signaling also plays a crucial role in T cells, e.g. in fine-tuning Th1 versus Th2 cell responses [39]. The consequences of spermidine treatment on cancer- vs. immune cell-associated NMDAR signaling have not been explored so far. Data from experiments with NMDAR agonists and antagonists, respectively, suggest a positive role of NMDAR activation on anticancer immunosurveillance but – at the same time – also on tumor progression: NMDAR antagonists elicit anticancer action through the inhibition of extracellular signal-regulated kinase 1/2 (ERK1/2) pathway and an upregulation of the tumor suppressor proteins p21 and p53 [38]. Vice versa, they also reduce T cell receptor signaling both in CD4+ and CD8+ T cells, resulting in decreased cytotoxic T lymphocyte action towards cancer cells [40]. NMDAR agonists shift Th cell populations towards Th2-like cells by promoting cell death predominantly in Th1-like cells [39].
–
The first findings on the anti-aging properties of spermidine implicated post-translational protein modifications in the beneficial effects of spermidine supplementation, particularly a reduction of protein lysine acetylation [41]. Indeed, spermidine treatment in colon carcinoma HCT116 cells decreases cellular protein acetylation [42], an effect attributed to the competitive inhibition of the protein acetyl-transferase EP300 [43]. In other settings, i.e., treatment of lymphocytic Jurkat cells, spermidine did not de-acetylate the EP300 targets histone 3 and autophagy-related protein 7 (ATG7) [4]. Intriguingly, somatic mutation data in bladder cancer indicates that nonsense and missense mutations of EP300 are associated with signatures of improved antitumor immunity, and overall favorable clinical prognosis [44]. Early reports suggested a tumor-suppressive function of EP300 [45], and at least in some cancer types, e.g. colorectal cancer, reduced EP300 expression is implicated in the attenuated expression of tumor-suppressive circular RNAs [46]. Still, accumulating evidence points at a oncogenic role in many scenarios, for instance in acute myeloid leukemia [47] or esophageal squamous carcinoma [48]. In line with these data, genetic knockdown of EP300 reduces metastatic capacity in xenograft mouse models of triple negative breast cancer and chemical inhibition of histone acetyltransferase activity increases sensitivity to radiotherapy in head and neck squamous cell carcinoma harboring potentially gain-of-function type mutations in EP300 [49].
–
In their recent paper, Al-Habsi et al. added another player to the spermidine target space: Mitochondrial trifunctional protein (MTP), an enzyme complex that mediates the enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and 3-ketoacyl-CoA thiolase activities of mitochondrial fatty acid oxidation (FAO) [2]. In detail, the authors demonstrate that spermidine allosterically activates MTP by binding to both HADHA and HADHB subunits, with a Kd of 0.10 μM (for HADHA). The low Michaelis-Menten constant (Km) of spermidine-mediated MTP activation (0.4-0.7 μM) compared to the substrate affinity of deoxyhypusine synthase (Km=6.49 µM) tempts the speculation that the activation of FAO might contribute to the acute effects of spermidine treatment. MTP activity is crucial for the energy demand of CD8+ T cells and accordingly, spermidine i.p. injections enhanced antitumor immunity [2]. Pharmacological MTP activation in the context of cancer should be carefully adjusted to the specific tumor bioenergetics, especially the reliance on anaerobic vs. oxidative energy metabolism. For instance, oxidative lung tumors can be treated with the MTP inhibitor trimetazidine, as they rely on FAO for ATP supply [50].
–
Covalent binding of spermidine
Spermidine is the sole substrate for the hypusination of eIF5A, a unique post-translational modification, which activates the protein [51]. Hypusinated eIF5A facilitates the translation of polyproline (and other sterically challenging) motifs and is required to express mitochondrial proteins efficiently [52]. Interfering with eIF5A hypusination severely impacts immune cells by reducing oxidative phosphorylation-dependent alternative activation of macrophages [52] and CD4+ helper T cell polarization [53]. Interestingly, aging-dependent B cell senescence has been linked to reduced spermidine levels and concomitant hypusination insufficiency [4]. In B cells, hypusination is required for transcription factor EB (TFEB) expression, which promotes lysosomal function and autophagy [4].
–
Beside the crucial role in immune cell function, eIF5A hypusination promotes neoplastic growth at multiple levels (reviewed in [54]), including several positive feedback loops: Multiplex gene expression analysis in colorectal cancer cells showed a feedback mechanism of hypusination on the expression of MYC (which indirectly augments hypusination by promoting polyamine biosynthesis). Hypusinated eIF5A resolves ribosomal stalling at distinct pausing motifs in the MYC coding sequence, and thereby promotes tumor growth [55]. The transcriptional coactivator Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) are additional factors that promote ODC expression. Hypusinated eIF5A supports the transcriptional repression of tumor suppressors by YAP/TAZ by enhancing the translation of lysine-specific histone demethylase 1 (LSD1), which demethylates histone H3 [56].
–
Translation control by spermidine (and other polyamines) is also vital for polyamine auto-regulation. Messenger RNAs of enzymes and regulators of polyamine biosynthesis have distinct sequence features that allow fine-tuning of protein synthesis based on polyamine levels [57]. Mechanistically, high polyamine levels modulate ribosomal scanning and frameshifting, likely by interfering with eIF5A function [58]. The role of this interference with eIF5A (as opposed to the positive regulation by increased hypusination) in the context of spermidine supplementation has yet to be investigated.
ANTI-CANCER EFFECTS OF SPERMIDINE VS. GROWTH-PROMOTING ACTIVITY
The tumor-promoting role of polyamine metabolism, which has been extensively reviewed elsewhere [10], demands a careful dissection of the risk profile of spermidine based on the available preclinical and clinical data: Lifelong supplementation of 3 mM spermidine to the drinking water did not lead to enhanced occurrence of tumors in male C57BL/6 mice [3], and i.p. injections of spermidine in mice with chemically provoked breast cancer did not show any difference in tumor mortality [59]. Furthermore, chemically induced liver cancer and fibrosis, as well as colon carcinogenesis, can be partially suppressed by simultaneous spermidine administration [60][61]. Spermidine injections enhance the antitumor efficacy of chemotherapy in mice in an autophagy-dependent fashion [14]. These preclinical findings are supported by prospective and case-control studies, indicating lower tumor-related mortality [11] and colorectal cancer risk [12][62] in individuals with higher dietary polyamine intake.
–
One possible explanation of the discrepancy between the anti-tumorigenic and tumor growth-promoting functions of spermidine might be the role of cell-autonomous vs. non-autonomous polyamine supply. We speculate that the anti-tumorigenic activity of spermidine is predominantly mediated in trans by improved immune responses. We thus speculate that intracellular spermidine production might promote proliferation, whereas external administration might activate the immune response in a fashion that overrides the tumor-promoting function (Figure 1). An important factor might be the administration route: While tumors might acutely benefit from enhanced polyamine biosynthesis in situ and elevated localized polyamine concentrations, a systemic administration might favor immune cell responses. Indeed, oral administration via the drinking water is sufficient to modulate polyamine-dependent pathways in B and T cells in mice [4][63]. Nevertheless, since clinical trials of spermidine supplementation in the context of tumor growth are lacking, spermidine supplementation, regardless of the administration route, is not advised for cancer patients at this point. Future research should address which tumor-types (with a special focus on tumor bioenergetics) and which genetic predisposition or microbiome of the host might modulate anti-tumorigenic or potential tumor growth-promoting functions of spermidine supplementation [64]. Also, unconventional approaches such as combining localized DFMO treatment with systemic spermidine supplementation should be explored.
–
ACUTE AND CHRONIC EFFECTS OF SPERMIDINE SUPPLEMENTATION
The pleiotropic target space of spermidine and the cell type-specific response to changes in polyamine metabolism pose questions about the acute and chronic effects of spermidine supplementation. As discussed above, MTP activation and the subsequent boost of FAO is a very early event in response to increased spermidine supply. Pathological scenarios involving disturbed lipid homeostasis might benefit from polyamine-targeted interventions. Previously, spermidine treatment was shown to ameliorate high-fat-diet-induced obesity via increased lipolysis in murine fat tissue [5]. Interestingly, these effects persisted in autophagy-incompetent mice, stimulating the speculation that MTP could be involved in these observations.
–
Interestingly, aged mice with heterozygous MTP deficiency develop hepatic steatosis, hyperinsulinemia and damaged hepatic mitochondria [65], signs of non-alcoholic fatty liver disease (NAFLD), one of the main risk factors for developing hepatocellular carcinoma [66]. In line, dysregulation of MTP is implicated in NAFLD pathogenesis in mice and humans [67][68] and, consequently, FAO is impaired in such scenarios [66]. This bidirectional dependence is pharmacologically targetable and relevant, as – for instance – methionine restriction increases FAO, along with augmented levels of MTP-subunits HADHA/HADHB, which elicits hepatoprotective effects in ob/ob mice [69].
–
At the same time, increased hypusination and likely EP300 inhibition, for which no Ki values are available, might be more relevant for the chronic effects of spermidine supplementation. Indeed, one hour of spermidine treatment suffices to elevate FAO in naïve CD8+ T cells isolated from aged mice [2] and the effects of spermidine supplementation on eIF5A hypusination and its consequences have been primarily studied in longer-lasting experimental designs (days in vitro [4][70], days to months in vivo [6][71]). This is biologically plausible, given that several enzymes (DHPS, DOHH) need to transduce spermidine levels into activated/hypusinated eIF5A and subsequent translational events. Thus, to delineate the hierarchy of these molecular players, the cascade of effects on MTP, FAO, hypusination, and acetylation events should be studied in vivo and selected cellular models in a timely resolved excursion spanning a few hours to multiple days or weeks (in case of in vivo supplementation).
–
A particularly interesting question is how the acute and chronic effects of spermidine interact. For instance, the molecular eIF5A axis was found impaired in mice and humans suffering from non-alcoholic steatohepatitis (NASH), another risk factor for liver cancer, in a recent publication [72]. The same authors found that spermidine feeding ameliorated liver damage in a diet-induced NASH mouse model by normalizing FAO and mitochondrial function [72]. While MTP was not investigated in this publication, spermidine improved FAO in an eIF5A-dependent manner. Previously, it was shown several times that the spermidine-hypusination axis critically regulates mitochondrial function [6][52][71] and immune cell development [73][74]. These findings raise interesting questions about the relationship between MTP and eIF5A. In a theoretical scenario, spermidine could acutely improve FAO via its interaction with MTP and maintain mitochondrial fatty acid utilization via eIF5A. However, this temporal hierarchy of events should be studied in coherent cellular and in vivo models.
CONCLUSION
In terms of chemical properties, spermidine is not a complex molecule, which has fostered a widespread belief in its functional simplicity [20]. However, it becomes increasingly evident that spermidine exhibits highly specific molecular interactions with multiple targets (Table 1), with dedicated downstream cellular and physiological effects. In the quest for molecular effectors of spermidine, we may abandon the idea that single factors mediate the various beneficial effects described in pre-clinical literature. Moreover, the relative contribution of spermidine-responsive pathways might differ depending on the cell type or tissue of interest (as well as on crucial aspects of experimental timing). This is particularly apparent in immune vs. cancer cells, where spermidine either improves anti-tumor immunity or promotes transcriptional and translational processes to facilitate proliferation, respectively.
–
Future studies should evaluate the relative effects of different targets (e.g., by employing specific inhibitors), and consider divergent response kinetics depending on the target, as well as cell type-specific effects. Here, a standard panel of functional readouts, at least for NMDAR activity (when appropriate), translation efficiency, autophagy, mitochondrial respiratory function, FAO, and hypusination, could help to harmonize experimental setups and improve our understanding of spermidine-mediated health benefits across experimental designs in cellular, pre-clinical and clinical studies.
REFERENCES
- Madeo F, Eisenberg T, Pietrocola F, Kroemer G (2018). Spermidine in health and disease. Science 359(6374): eaan2788. 10.1126/science.aan2788
- Al-Habsi M, Chamoto K, Matsumoto K, Nomura N, Zhang B, Sugiura Y, Sonomura K, Maharani A, Nakajima Y, Wu Y, Nomura Y, Menzies R, Tajima M, Kitaoka K, Haku Y, Delghandi S, Yurimoto K, Matsuda F, Iwata S, Ogura T, Fagarasan S, Honjo T (2022). Spermidine activates mitochondrial trifunctional protein and improves antitumor immunity in mice. Science 378(6618): eabj3510. 10.1126/science.abj3510
- Eisenberg T et al. (2016). Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med 22(12): 1428–1438. 10.1038/nm.4222
- Zhang H, Alsaleh G, Feltham J, Sun Y, Napolitano G, Riffelmacher T, Charles P, Frau L, Hublitz P, Yu Z, Mohammed S, Ballabio A, Balabanov S, Mellor J, Simon AK (2019). Polyamines Control eIF5A Hypusination, TFEB Translation, and Autophagy to Reverse B Cell Senescence. Mol Cell 76(1): 110-125.e9. 10.1016/j.molcel.2019.08.005
- Liao CY, Kummert OMP, Bair AM, Alavi N, Alavi J, Miller DM, Bagga I, Schempf AM, Hsu YM, Woods BD, Brown Mayfield SM, Mitchell AN, Tannady G, Talbot AR, Dueck AM, Barrera Ovando R, Parker HD, Wang J, Schoeneweis JK, Kennedy BK (2021). The Autophagy Inducer Spermidine Protects Against Metabolic Dysfunction During Overnutrition. J Gerontol Ser A 76(10): 1714–1725. 10.1093/gerona/glab145
- Schroeder S et al. (2021). Dietary spermidine improves cognitive function. Cell Rep 35(2): 108985. 10.1016/j.celrep.2021.108985
- Hofer SJ, Simon AK, Bergmann M, Eisenberg T, Kroemer G, Madeo F (2022). Mechanisms of spermidine-induced autophagy and geroprotection. Nat Aging 2(12): 1112–1129. 10.1038/s43587-022-00322-9
- Phanstiel IV O (2018). An overview of polyamine metabolism in pancreatic ductal adenocarcinoma. Int J Cancer 142(10): 1968–1976. 10.1002/ijc.31155
- Murray-Stewart TR, Woster PM, Casero RA Jr (2016). Targeting polyamine metabolism for cancer therapy and prevention. Biochem J 473(19): 2937–2953. 10.1042/BCJ20160383
- Casero RA Jr, Murray Stewart T, Pegg AE (2018). Polyamine metabolism and cancer: treatments, challenges and opportunities. Nat Rev Cancer 18(11): 681–695. 10.1038/s41568-018-0050-3
- Kiechl S, Pechlaner R, Willeit P, Notdurfter M, Paulweber B, Willeit K, Werner P, Ruckenstuhl C, Iglseder B, Weger S, Mairhofer B, Gartner M, Kedenko L, Chmelikova M, Stekovic S, Stuppner H, Oberhollenzer F, Kroemer G, Mayr M, Eisenberg T, Tilg H, Madeo F, Willeit J (2018). Higher spermidine intake is linked to lower mortality: a prospective population-based study. Am J Clin Nutr 108(2): 371–380. 10.1093/ajcn/nqy102
- Huang CY, Fang YJ, Abulimiti A, Yang X, Li L, Liu KY, Zhang X, Feng XL, Chen YM, Zhang CX (2020). Dietary Polyamines Intake and Risk of Colorectal Cancer: A Case-Control Study. Nutrients 12(11): 3575. 10.3390/nu12113575
- Wang L et al. (2023). Spermine enhances antiviral and anticancer responses by stabilizing DNA binding with the DNA sensor cGAS. Immunity 56(2): 272-288.e7. 10.1016/j.immuni.2023.01.001
- Wang L, Li S, Wang K, Wang N, Liu Q, Sun Z, Wang L, Wang L, Liu Q, Song C, Yang Q (2016). Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell 30(1): 147–160. 10.1016/j.ccell.2016.05.016
- Watanabe S, Kusama-Eguchi K, Kobayashi H, Igarashi K (1991). Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J Biol Chem 266(31): 20803–20809. 10.1016/s0021-9258(18)54780-3
- Rowatt E, Williams RJ (1992). The binding of polyamines and magnesium to DNA. J Inorg Biochem 46(2): 87–97. 10.1016/0162-0134(92)80012-K
- Thomas TJ, Bloomfield VA (1984). Ionic and structural effects on the thermal helix-coil transition of DNA complexed with natural and synthetic polyamines. Biopolymers 23(7): 1295–1306. 10.1002/bip.360230713
- Perepelytsya S, Uličný J, Laaksonen A, Mocci F (2019). Pattern preferences of DNA nucleotide motifs by polyamines putrescine2+, spermidine3+ and spermine4+. Nucleic Acids Res 47(12): 6084–6097. 10.1093/nar/gkz434
- Winther KS, Sørensen MA, Svenningsen SL (2021). Polyamines are Required for tRNA Anticodon Modification in Escherichia coli. J Mol Biol 433(15): 167073. 10.1016/j.jmb.2021.167073
- Lightfoot HL, Hall J (2014). Endogenous polyamine function—the RNA perspective. Nucleic Acids Res 42(18): 11275–11290. 10.1093/nar/gku837
- Khan AU, Mei YH, Wilson T (1992). A proposed function for spermine and spermidine: protection of replicating DNA against damage by singlet oxygen. Proc Natl Acad Sci 89(23): 11426–11427. 10.1073/pnas.89.23.11426
- Su WH, Chan CET, Lian T, Biju M, Miura A, Alkhafaji SA, Do KK, Latifi B, Nguyen TT, Schriner SE (2021). Protection of nuclear DNA by lifespan-extending compounds in the yeast Saccharomyces cerevisiae. Mutat Res Mol Mech Mutagen 822: 111738. 10.1016/j.mrfmmm.2021.111738
- Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA Jr (1998). The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci 95(19): 11140–11145. 10.1073/pnas.95.19.11140
- Das KC, Misra HP (2004). Hydroxyl radical scavenging and singlet oxygen quenching properties of polyamines. Mol Cell Biochem 262(1/2): 127–133. 10.1023/B:MCBI.0000038227.91813.79
- Panagiotidis CA, Artandi S, Calame K, Silverstein SJ (1995). Polyamines alter sequence-specific DNA-protein interactions. Nucleic Acids Res 23(10): 1800–1809. 10.1093/nar/23.10.1800
- Shah N, Thomas T, Shirahata A, Sigal LH, Thomas TJ (1999). Activation of nuclear factor kappaB by polyamines in breast cancer cells. Biochemistry 38(45): 14763–14774. 10.1021/bi991291v
- Lindemose S, Nielsen PE, Møllegaard NE (2005). Polyamines preferentially interact with bent adenine tracts in double-stranded DNA. Nucleic Acids Res 33(6): 1790–1803. 10.1093/nar/gki319
- Maddox J, Shakya A, South S, Shelton D, Andersen JN, Chidester S, Kang J, Gligorich KM, Jones DA, Spangrude GJ, Welm BE, Tantin D (2012). Transcription factor Oct1 is a somatic and cancer stem cell determinant. PLoS Genet 8(11): e1003048. 10.1371/journal.pgen.1003048
- Schuster I, Bernhardt R (2011). Interactions of natural polyamines with mammalian proteins. Biomol Concepts 2(1–2): 79–94. 10.1515/bmc.2011.007
- Davis RA, Vullo D, Supuran CT, Poulsen SA (2014). Natural product polyamines that inhibit human carbonic anhydrases. BioMed Res Int 2014: 374079. 10.1155/2014/374079
- Carta F, Temperini C, Innocenti A, Scozzafava A, Kaila K, Supuran CT (2010). Polyamines inhibit carbonic anhydrases by anchoring to the zinc-coordinated water molecule. J Med Chem 53(15): 5511–5522. 10.1021/jm1003667
- Chiche J, Ilc K, Laferrière J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouysségur J (2009). Hypoxia-Inducible Carbonic Anhydrase IX and XII Promote Tumor Cell Growth by Counteracting Acidosis through the Regulation of the Intracellular pH. Cancer Res 69(1): 358–368. 10.1158/0008-5472.CAN-08-2470
- Miska J, Rashidi A, Lee-Chang C, Gao P, Lopez-Rosas A, Zhang P, Burga R, Castro B, Xiao T, Han Y, Hou D, Sampat S, Cordero A, Stoolman JS, Horbinski CM, Burns M, Reshetnyak YK, Chandel NS, Lesniak MS (2021). Polyamines drive myeloid cell survival by buffering intracellular pH to promote immunosuppression in glioblastoma. Sci Adv 7(8): eabc8929. 10.1126/sciadv.abc8929
- Ransom RW, Stec NL (1988). Cooperative Modulation of [ 3 H]MK-801 Binding to the N-Methyl-d-Aspartate Receptor-Ion Channel Complex by l-Glutamate, Glycine, and Polyamines. J Neurochem 51(3): 830–836. 10.1111/j.1471-4159.1988.tb01818.x
- Sacaan AI, Johnson KM (1990). Spermidine reverses arcaine's inhibition of N-methyl-D-aspartate-induced hippocampal [3H]norepinephrine release. J Pharmacol Exp Ther 255(3): 1060–1063. 2148186.
- Subramaniam S, O'Connor MJ, Masukawa LM, McGonigle P (1994). Polyamine Effects on the NMDA Receptor in Human Brain. Exp Neurol 130(2): 323–330. 10.1006/exnr.1994.1210
- Mony L, Zhu S, Carvalho S, Paoletti P (2011). Molecular basis of positive allosteric modulation of GluN2B NMDA receptors by polyamines. EMBO J 30(15): 3134–3146. 10.1038/emboj.2011.203
- Stepulak A, Sifringer M, Rzeski W, Endesfelder S, Gratopp A, Pohl EE, Bittigau P, Felderhoff-Mueser U, Kaindl AM, Bührer C, Hansen HH, Stryjecka-Zimmer M, Turski L, Ikonomidou C (2005). NMDA antagonist inhibits the extracellular signal-regulated kinase pathway and suppresses cancer growth. Proc Natl Acad Sci U S A 102(43): 15605–15610. 10.1073/pnas.0507679102
- Orihara K, Odemuyiwa SO, Stefura WP, Ilarraza R, HayGlass KT, Moqbel R (2018). Neurotransmitter signalling via NMDA receptors leads to decreased T helper type 1-like and enhanced T helper type 2-like immune balance in humans. Immunology 153(3): 368–379. 10.1111/imm.12846
-
Kahlfuβ S, Simma N, Mankiewicz J, Bose T, Lowinus T, Klein-Hessling S, Sprengel R, Schraven B, Heine M, Bommhardt U (2014). Immunosuppression by N -Methyl-
d -Aspartate Receptor Antagonists Is Mediated through Inhibition of K v 1.3 and K Ca 3.1 Channels in T Cells. Mol Cell Biol 34(5): 820–831. 10.1128/MCB.01273-13 - Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Fröhlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F (2009). Induction of autophagy by spermidine promotes longevity. Nat Cell Biol 11(11): 1305–1314. 10.1038/ncb1975
- Morselli E, Mariño G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, Cabrera S, Bénit P, Rustin P, Criollo A, Kepp O, Galluzzi L, Shen S, Malik SA, Maiuri MC, Horio Y, López-Otín C, Andersen JS, Tavernarakis N, Madeo F, Kroemer G (2011). Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol 192(4): 615–629. 10.1083/jcb.201008167
- Pietrocola F, Lachkar S, Enot DP, Niso-Santano M, Bravo-San Pedro JM, Sica V, Izzo V, Maiuri MC, Madeo F, Mariño G, Kroemer G (2015). Spermidine induces autophagy by inhibiting the acetyltransferase EP300. Cell Death Differ 22(3): 509–516. 10.1038/cdd.2014.215
- Zhu G, Pei L, Li Y, Gou X (2020). EP300 mutation is associated with tumor mutation burden and promotes antitumor immunity in bladder cancer patients. Aging 12(3): 2132–2141. 10.18632/aging.102728
- Gayther SA, Batley SJ, Linger L, Bannister A, Thorpe K, Chin SF, Daigo Y, Russell P, Wilson A, Sowter HM, Delhanty JD, Ponder BA, Kouzarides T, Caldas C (2000). Mutations truncating the EP300 acetylase in human cancers. Nat Genet 24(3): 300–303. 10.1038/73536
- Ding N, You AB, Yang H, Hu GS, Lai CP, Liu W, Ye F (2023). A Tumor-suppressive Molecular Axis EP300/circRERE/miR-6837-3p/MAVS Activates Type I IFN Pathway and Antitumor Immunity to Suppress Colorectal Cancer. Clin Cancer Res 29(11): 2095–2109. 10.1158/1078-0432.CCR-22-3836
- Pan F, Iwasaki M, Wu W, Jiang Y, Yang X, Zhu L, Zhao Z, Cleary ML (2023). Enhancer remodeling drives MLL oncogene-dependent transcriptional dysregulation in leukemia stem cells. Blood Adv 7(11): 2504–2519. 10.1182/bloodadvances.2022008787
- Bi Y, Kong P, Zhang L, Cui H, Xu X, Chang F, Yan T, Li J, Cheng C, Song B, Niu X, Liu X, Liu X, Xu E, Hu X, Qian Y, Wang F, Li H, Ma Y, Yang J, Liu Y, Zhai Y, Wang Y, Zhang Y, Liu H, Liu J, Wang J, Cui Y, Cheng X (2019). EP300 as an oncogene correlates with poor prognosis in esophageal squamous carcinoma. J Cancer 10(22): 5413–5426. 10.7150/jca.34261
- Kumar M, Molkentine D, Molkentine J, Bridges K, Xie T, Yang L, Hefner A, Gao M, Bahri R, Dhawan A, Frederick MJ, Seth S, Abdelhakiem M, Beadle BM, Johnson F, Wang J, Shen L, Heffernan T, Sheth A, Ferris RL, Myers JN, Pickering CR, Skinner HD (2021). Inhibition of histone acetyltransferase function radiosensitizes CREBBP/EP300 mutants via repression of homologous recombination, potentially targeting a gain of function. Nat Commun 12(1): 6340. 10.1038/s41467-021-26570-8
- Amoedo ND, Sarlak S, Obre E, Esteves P, Bégueret H, Kieffer Y, Rousseau B, Dupis A, Izotte J, Bellance N, Dard L, Redonnet-Vernhet I, Punzi G, Rodrigues MF, Dumon E, Mafhouf W, Guyonnet-Dupérat V, Gales L, Palama T, Bellvert F, Dugot-Senan N, Claverol S, Baste JM, Lacombe D, Rezvani HR, Pierri CL, Mechta-Grigoriou F, Thumerel M, Rossignol R (2021). Targeting the mitochondrial trifunctional protein restrains tumor growth in oxidative lung carcinomas. J Clin Invest 131(1): e133081, 133081. 10.1172/JCI133081
- Park MH, Cooper HL, Folk JE (1981). Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc Natl Acad Sci 78(5): 2869–2873. 10.1073/pnas.78.5.2869
- Puleston DJ et al. (2019). Polyamines and eIF5A Hypusination Modulate Mitochondrial Respiration and Macrophage Activation. Cell Metab 30(2): 352-363.e8. 10.1016/j.cmet.2019.05.003
- Puleston DJ, Baixauli F, Sanin DE, Edwards-Hicks J, Villa M, Kabat AM, Kamiński MM, Stanckzak M, Weiss HJ, Grzes KM, Piletic K, Field CS, Corrado M, Haessler F, Wang C, Musa Y, Schimmelpfennig L, Flachsmann L, Mittler G, Yosef N, Kuchroo VK, Buescher JM, Balabanov S, Pearce EJ, Green DR, Pearce EL (2021). Polyamine metabolism is a central determinant of helper T cell lineage fidelity. Cell 184(16): 4186-4202.e20. 10.1016/j.cell.2021.06.007
- Mathews MB, Hershey JW (2015). The translation factor eIF5A and human cancer. Biochim Biophys Acta BBA – Gene Regul Mech 1849(7): 836–844. 10.1016/j.bbagrm.2015.05.002
- Coni S, Serrao SM, Yurtsever ZN, Di Magno L, Bordone R, Bertani C, Licursi V, Ianniello Z, Infante P, Moretti M, Petroni M, Guerrieri F, Fatica A, Macone A, De Smaele E, Di Marcotullio L, Giannini G, Maroder M, Agostinelli E, Canettieri G (2020). Blockade of EIF5A hypusination limits colorectal cancer growth by inhibiting MYC elongation. Cell Death Dis 11(12): 1045. 10.1038/s41419-020-03174-6
- Li H, Wu BK, Kanchwala M, Cai J, Wang L, Xing C, Zheng Y, Pan D (2022). YAP/TAZ drives cell proliferation and tumour growth via a polyamine–eIF5A hypusination–LSD1 axis. Nat Cell Biol 24(3): 373–383. 10.1038/s41556-022-00848-5
- Dever TE, Ivanov IP (2018). Roles of polyamines in translation. J Biol Chem 293(48): 18719–18729. 10.1074/jbc.TM118.003338
- Ivanov IP, Shin BS, Loughran G, Tzani I, Young-Baird SK, Cao C, Atkins JF, Dever TE (2018). Polyamine Control of Translation Elongation Regulates Start Site Selection on Antizyme Inhibitor mRNA via Ribosome Queuing. Mol Cell 70(2): 254-264.e6. 10.1016/j.molcel.2018.03.015
- Buqué A, Bloy N, Perez-Lanzón M, Iribarren K, Humeau J, Pol JG, Levesque S, Mondragon L, Yamazaki T, Sato A, Aranda F, Durand S, Boissonnas A, Fucikova J, Senovilla L, Enot D, Hensler M, Kremer M, Stoll G, Hu Y, Massa C, Formenti SC, Seliger B, Elemento O, Spisek R, André F, Zitvogel L, Delaloge S, Kroemer G, Galluzzi L (2020). Immunoprophylactic and immunotherapeutic control of hormone receptor-positive breast cancer. Nat Commun 11(1): 3819. 10.1038/s41467-020-17644-0
- Yu L, Lu M, Jia D, Ma J, Ben-Jacob E, Levine H, Kaipparettu BA, Onuchic JN (2017). Modeling the Genetic Regulation of Cancer Metabolism: Interplay between Glycolysis and Oxidative Phosphorylation. Cancer Res 77(7): 1564–1574. 10.1158/0008-5472.CAN-16-2074
- Gobert AP, Latour YL, Asim M, Barry DP, Allaman MM, Finley JL, Smith TM, McNamara KM, Singh K, Sierra JC, Delgado AG, Luis PB, Schneider C, Washington MK, Piazuelo MB, Zhao S, Coburn LA, Wilson KT (2022). Protective Role of Spermidine in Colitis and Colon Carcinogenesis. Gastroenterology 162(3): 813-827.e8. 10.1053/j.gastro.2021.11.005
- Vargas AJ, Ashbeck EL, Wertheim BC, Wallace RB, Neuhouser ML, Thomson CA, Thompson PA (2015). Dietary polyamine intake and colorectal cancer risk in postmenopausal women. Am J Clin Nutr 102(2): 411–419. 10.3945/ajcn.114.103895
- Carriche GM, Almeida L, Stüve P, Velasquez L, Dhillon-LaBrooy A, Roy U, Lindenberg M, Strowig T, Plaza-Sirvent C, Schmitz I, Lochner M, Simon AK, Sparwasser T (2021). Regulating T-cell differentiation through the polyamine spermidine. J Allergy Clin Immunol 147(1): 335-348.e11. 10.1016/j.jaci.2020.04.037
- Hofer SJ, Kroemer G, Kepp O (2022). Autophagy-inducing nutritional interventions in experimental and clinical oncology. Int Rev Cell Mol Biol 373: 125–158. 10.1016/bs.ircmb.2022.08.003
- Ibdah JA, Perlegas P, Zhao Y, Angdisen J, Borgerink H, Shadoan MK, Wagner JD, Matern D, Rinaldo P, Cline JM (2005). Mice heterozygous for a defect in mitochondrial trifunctional protein develop hepatic steatosis and insulin resistance. Gastroenterology 128(5): 1381–1390. 10.1053/j.gastro.2005.02.001
- Moore MP, Cunningham RP, Meers GM, Johnson SA, Wheeler AA, Ganga RR, Spencer NM, Pitt JB, Diaz-Arias A, Swi AIA, Hammoud GM, Ibdah JA, Parks EJ, Rector RS (2022). Compromised hepatic mitochondrial fatty acid oxidation and reduced markers of mitochondrial turnover in human NAFLD. Hepatol Baltim Md 76(5): 1452–1465. 10.1002/hep.32324
- Guo L, Zhou SR, Wei XB, Liu Y, Chang XX, Liu Y, Ge X, Dou X, Huang HY, Qian SW, Li X, Lei QY, Gao X, Tang QQ (2016). Acetylation of Mitochondrial Trifunctional Protein α-Subunit Enhances Its Stability To Promote Fatty Acid Oxidation and Is Decreased in Nonalcoholic Fatty Liver Disease. Mol Cell Biol 36(20): 2553–2567. 10.1128/MCB.00227-16
- Nassir F, Arndt JJ, Johnson SA, Ibdah JA (2018). Regulation of mitochondrial trifunctional protein modulates nonalcoholic fatty liver disease in mice. J Lipid Res 59(6): 967–973. 10.1194/jlr.M080952
- Malloy VL, Perrone CE, Mattocks DA, Ables GP, Caliendo NS, Orentreich DS, Orentreich N (2013). Methionine restriction prevents the progression of hepatic steatosis in leptin-deficient obese mice. Metabolism 62(11): 1651–1661. 10.1016/j.metabol.2013.06.012
- Alsaleh G, Panse I, Swadling L, Zhang H, Richter FC, Meyer A, Lord J, Barnes E, Klenerman P, Green C, Simon AK (2020). Autophagy in T cells from aged donors is maintained by spermidine and correlates with function and vaccine responses. eLife 9: e57950. 10.7554/eLife.57950
- Liang Y, Piao C, Beuschel CB, Toppe D, Kollipara L, Bogdanow B, Maglione M, Lützkendorf J, See JCK, Huang S, Conrad TOF, Kintscher U, Madeo F, Liu F, Sickmann A, Sigrist SJ (2021). eIF5A hypusination, boosted by dietary spermidine, protects from premature brain aging and mitochondrial dysfunction. Cell Rep 35(2): 108941. 10.1016/j.celrep.2021.108941
- Zhou J, Pang J, Tripathi M, Ho JP, Widjaja AA, Shekeran SG, Cook SA, Suzuki A, Diehl AM, Petretto E, Singh BK, Yen PM (2022). Spermidine-mediated hypusination of translation factor EIF5A improves mitochondrial fatty acid oxidation and prevents non-alcoholic steatohepatitis progression. Nat Commun 13(1): 5202. 10.1038/s41467-022-32788-x
- Puleston DJ, Zhang H, Powell TJ, Lipina E, Sims S, Panse I, Watson AS, Cerundolo V, Townsend AR, Klenerman P, Simon AK (2014). Autophagy is a critical regulator of memory CD8+ T cell formation. eLife 3: e03706. 10.7554/eLife.03706
- Wagner A, Wang C, Fessler J, DeTomaso D, Avila-Pacheco J, Kaminski J, Zaghouani S, Christian E, Thakore P, Schellhaass B, Akama-Garren E, Pierce K, Singh V, Ron-Harel N, Douglas VP, Bod L, Schnell A, Puleston D, Sobel RA, Haigis M, Pearce EL, Soleimani M, Clish C, Regev A, Kuchroo VK, Yosef N (2021). Metabolic modeling of single Th17 cells reveals regulators of autoimmunity. Cell 184(16): 4168-4185.e21. 10.1016/j.cell.2021.05.045
- Abid MR, Sasaki K, Titani K, Miyazaki M (1997). Biochemical and Immunological Characterization of Deoxyhypusine Synthase Purified from the Yeast Saccharomyces carlsbergensis. J Biochem 121(4): 769–778. 10.1093/oxfordjournals.jbchem.a021652
–
AUTHOR CONTRIBUTIONS
A.Z. and S.J.H. wrote the first draft. All authors conceptu-alized the minireview, proofread, edited, and contribut-ed substantially to the final version.
ACKNOWLEDGMENTS
F.M. is grateful to the Austrian Sci-ence Fund FWF (F3012, W1226, P29203, P29262, P27893, P31727, P37016) and the Austrian Federal Min-istry of Education, Science and Research as well as the University of Graz for grants ‘Unkonventionelle For-schung-InterFast and Fast4Health’ and ‘flysleep’ (BMWFW-80.109/0001-WF/V/3b/2015). We acknowledge the support of the Field of Excellence Bio-Health of NAWI Graz and the BioTechMed-Graz flagship project ‘EPIAge’.
COPYRIGHT
© 2023

Molecular targets of spermidine: implications for cancer suppression by Zimmermann et al. is licensed under a Creative Commons Attribution 4.0 International License.