Back to article: Endolysosomal dysfunction and exosome secretion: implications for neurodegenerative disorders
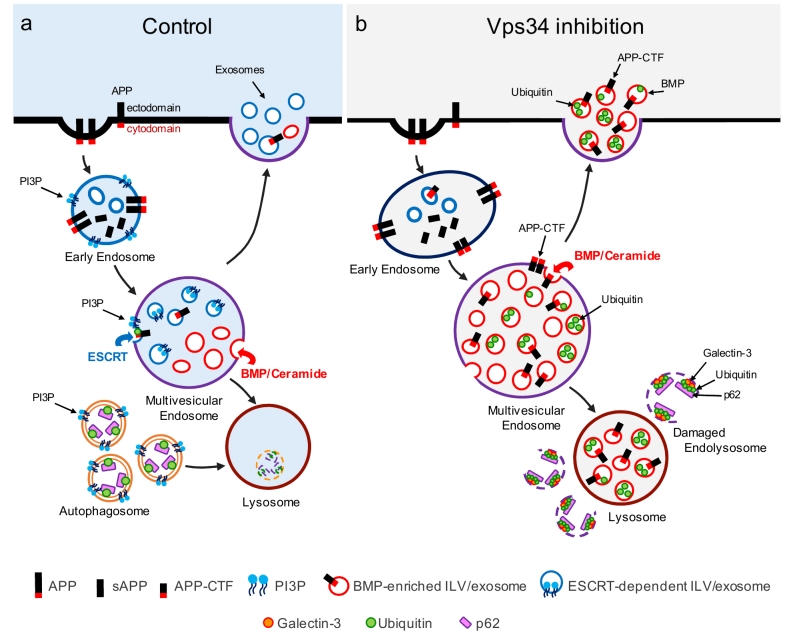
FIGURE 1: Vps34 inhibition causes endolysosomal dysfunction and enhanced release of atypical exosomes harboring poly-ubiquitinated proteins, APP-CTFs and the atypical phospholipid BMP. (a) In control cells, newly synthesized APP traffics to the plasma membrane (not shown), where it undergoes clathrin-mediated endocytosis and sorting into PI3P-enriched early endosomes. The acidic endosomal lumen is optimal for amyloidogenic processing by BACE1. APP-CTFs and a pool of full length APP are sorted to intraluminal vesicles (ILVs) of multivesicular endosomes (MVEs) via the ESCRT pathway, which requires PI3P and ubiquitination of lysine residues of the APP cytodomain. APP-CTFs can then be processed by g-secretase into amyloid-b (Ab), degraded in lysosomes or secreted in exosomes upon fusion of MVEs with the plasma membrane. Of note, PI3P- and BMP-positive ILVs are segregated from each other within MVEs and, under control conditions, exosomes are largely devoid of BMP. In a separate pathway, autophagosome formation and maturation also requires PI3P and mediate clearance of cytosolic cargoes and damaged organelles labelled with autophagy receptors (e.g., p62) upon their fusion with the endolysosomal system. (b) Vps34 inhibition causes enlargement of Rab5-positive early endosomes which are enriched for endosomal adaptor APPL1 instead of PI3P-binding protein, EEA1 (not shown). Through pleiotropic effects on the endolysosomal system, Vps34 inhibition causes accumulation of proteins and lipids in MVEs and lysosomes, resulting in a fraction of them undergoing physical damage, as denoted by the enrichment of galectin-3 on p62- and ubiquitin-positive structures. While PI3P deficiency reduces ESCRT-dependent sorting of cargoes, including APP, into ILVs, local synthesis of ceramide by neutral sphingomyelinase 2 facilitates ILV sorting and secretion of poly-ubiquitinated proteins, APP-CTFs and BMP in exosomes, alleviating lysosomal burden. BMP represents a small fraction (< 5%) of phospholipids measured in total exosome derived from N2a cells treated with the Vps34 inhibitor, compared to more abundant phospholipids, such as phosphatidylcholine (PC) (~30%), phosphatidylserine and phosphatidylethan-olamine (PE) plasmalogen (~20% each), PE and ether PC (~10% each), although we predict it may be significantly enriched on a subset of exosomes.