Back to article: Foie gras and liver regeneration: a fat dilemma
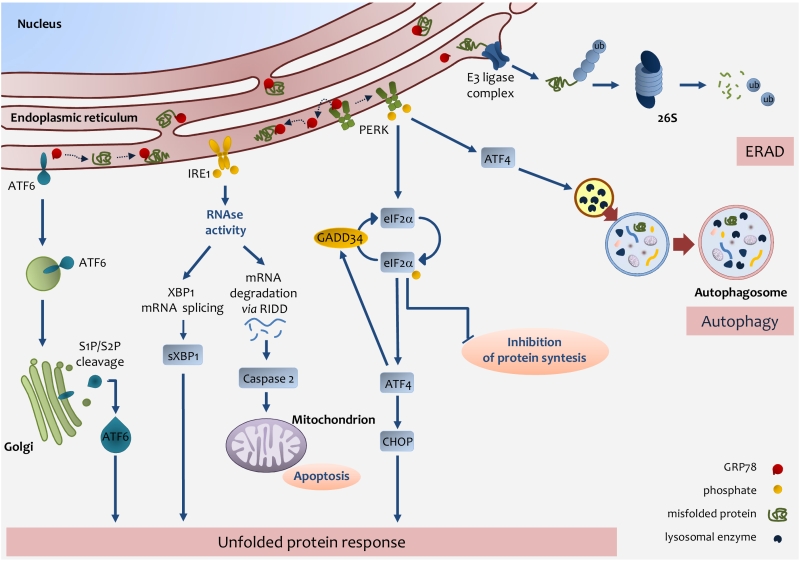
FIGURE 2: Endoplasmic reticulum (ER) stress pathways. ER stress occurs through the accu-mulation of misfolded proteins in the ER lumen. The role of the UPR is to re-establish ER homeostasis by reducing de novo protein synthesis and improving the folding and clearance capacity of the ER. The first mechanism against ER stress is ERAD, by which misfolded proteins are translocated back to the cytosol, ubiquitylated and degraded by the proteasome. When the level of misfolded proteins is too high, the cell activates UPR consisting in the activation of three transmembrane pathways. The main effectors are PERK, IRE1 and ATF6. Effectors activation is initiated by the removal of GRP78 allowing the translocation of the latter from the ER membrane to the ER lumen where it associates with unfolded proteins. eIF2α phosphorylation inhibits protein translation except for ATF4 which induces the expression of factors involved in antioxidant defence, amino acid metabolism, autophagy and apoptosis, such as CHOP. ATF4 also induces the expression of GADD34, which expression enables the dephosphorylation of eIF2α and the re-initiation of translation. PERK-mediated induction of ATF4, can also promote the expression of key autophagy-related proteins, which are needed for autophagosome formation. Once activated, IRE1 promotes the splicing of XBP1 mRNA, which is then translated into the active sXBP1, which transactivates the expression of components related to protein folding, ERAD and protein quality control. IRE1 also promotes the degradation of RNAs localized in the ER vicinity by regulated IRE1-dependent decay (RIDD), which induces caspase-2 and mitochondrial apoptosis. Upon GRP78 release, ATF6 is transferred to the Golgi apparatus as inactive precursors and cleaved by membrane-bound site-1 (S1P) and site-2 (S2P) proteases into an active form, which induces the expression of chaperones and UPR components. IRE1-mediated activation of XBP1 as well as ATF6 activation induces the expression of chaperones and UPR components.