Back to article: Mitochondrial dysfunction and its role in tissue-specific cellular stress
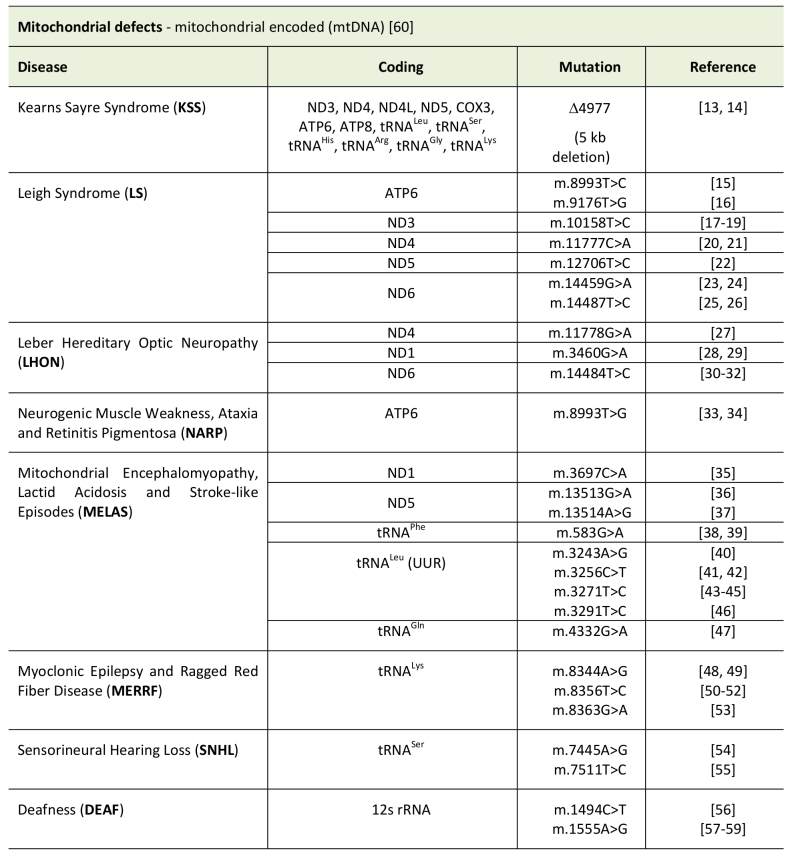
TABLE 1. Types of mitochondrial disease caused by mitochondrial encoded genes.
13. Zeviani M, Moraes CT, DiMauro S, Nakase H, Bonilla E, Schon EA, and Rowland LP (1988). Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology 38(8): 1339–1339. http://dx.doi.org/10.1212/WNL.38.8.1339
14. Lestienne P, and Ponsot G (1988). KEARNS-SAYRE SYNDROME WITH MUSCLE MITOCHONDRIAL DNA DELETION. The Lancet 331(8590): 885. http://dx.doi.org/10.1016/S0140-6736(88)91632-7
15. de Vries DD, van Engelen BGM, Gabreëls FJM, Ruitenbeek W, and van Oost BA (2004). A second missense mutation in the mitochondrial ATPase 6 gene in Leigh’s syndrome. Ann Neurol 34(3): 410–412. http://dx.doi.org/10.1002/ana.410340319
16. Carrozzo R, Murray J, Santorelli FM, and Capaldi RA (2000). The T9176G mutation of human mtDNA gives a fully assembled but inactive ATP synthase when modeled in Escherichia coli. FEBS Lett 486(3): 297–299. http://dx.doi.org/10.1016/S0014-5793(00)02244-4
17. Crimi M, Papadimitriou A, Galbiati S, Palamidou P, Fortunato F, Bordoni A, Papandreou U, Papadimitriou D, Hadjigeorgiou GM, Drogari E, Bresolin N, and Comi GP (2004). A New Mitochondrial DNA Mutation in ND3 Gene Causing Severe Leigh Syndrome with Early Lethality. Pediatric Res 486(3): 297–299. http://dx.doi.org/10.1203/01.PDR.0000117844.73436.68
18. McFarland R, Kirby DM, Fowler KJ, Ohtake A, Ryan MT, Amor DJ, Fletcher JM, Dixon JW, Collins FA, Turnbull DM, Taylor RW, and Thor-burn DR (2004). De novo mutations in the mitochondrial ND3 gene as a cause of infantile mitochondrial encephalopathy and complex I deficiency. Ann Neurol 55(1): 58–64. http://dx.doi.org/10.1002/ana.10787
19. Lebon S, Chol M, Benit P, Mugnier C, Chretien D, Giurgea I, Kern I, Girardin E, Hertz-Pannier L, de Lonlay P, Rötig A, Rustin P, and Munnich A (2003). Recurrent de novo mitochondrial DNA mutations in respiratory chain deficiency. J Med Genet 40(12): 896–899. http://dx.doi.org/10.1136/jmg.40.12.8967
20. Deschauer M, Bamberg C, Claus D, Zierz S, Turnbull DM, and Taylor RW (2003). Late-onset encephalopathy associated with a C11777A mutation of mitochondrial DNA Neurology 60(8): 1357–1359. http://dx.doi.org/10.1212/01.WNL.0000055869.99975.4B
21. Komaki H, Akanuma J, Iwata H, Takahashi T, Mashima Y, Nonaka I, and Goto Y-I (2003). A novel mtDNA C11777A mutation in Leigh syndrome. Mitochondrion 2(4): 293–304. http://dx.doi.org/10.1016/S1567-7249(03)00003-5
22. Taylor RW, Morris AA, Hutchinson M, and Turnbull DM (2002). Leigh disease associated with a novel mitochondrial DNA ND5 mutation. Eur J Hum Genet 10(2): 141–144. http://dx.doi.org/10.1038/sj.ejhg.5200773
23. Kirby DM, Kahler SG, Freckmann ML, Reddihough D, and Thorburn DR (2000). Leigh disease caused by the mitochondrial DNA G14459A mutation in unrelated families. Ann Neurol 48(1): 102–104. http://dx.doi.org/10.1073/pnas.91.13.6206
25. Solano A, Roig M, Vives-Bauza C, Hernandez-Peña J, Garcia-Arumi E, Playan A, López-Pérez MJ, Andreu AL, and Montoya J (2003). Bilateral striatal necrosis associated with a novel mutation in the mitochondrial ND6 gene. Ann Neurol 54(4): 527–530. http://dx.doi.org/10.1002/ana.10682
26. Ugalde C, Triepels RH, Coenen MJH, Van Den Heuvel LP, Smeets R, Uusimaa J, Briones P, Campistol J, Majamaa K, Smeitink JAM, and Nijtmans LGJ (2003). Impaired complex I assembly in a Leigh syndrome patient with a novel missense mutation in the ND6 gene. Ann Neurol 54(5): 665–669. http://dx.doi.org/10.1002/ana.107342
27. Wallace D, Singh G, Lott M, Hodge J, Schurr T, Lezza A, Elsas L, and Nikoskelainen E (1988). Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science 242(4884): 1427–1430. http://dx.doi.org/10.1126/science.3201231
28. Howell N, Bindoff LA, McCullough DA, Kubacka I, Poulton J, Mackey D, Taylor L, and Turnbull DM (1991). Leber hereditary optic neuropathy: identification of the same mitochondrial ND1 mutation in six pedigrees. Am J Hum Genet 49(5): 939–950. https://www.ncbi.nlm.nih.gov/pubmed/?term=1928099
29. Huoponen K, Vilkki J, Aula P, Nikoskelainen EK, and Savontaus ML (1991). A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy. Am J Hum Genet 48(6): 1147–1153. https://www.ncbi.nlm.nih.gov/pubmed/?term=1674640
30. Brown MD, Voljavec AS, Lott MT, MacDonald I, and Wallace DC (1992). Leber’s hereditary optic neuropathy: a model for mitochondrial neurodegenerative diseases. FASEB J 6(10): 2791–2799. https://www.ncbi.nlm.nih.gov/pubmed/?term=1634041
31. Howell N, Kubacka I, Xu M, and McCullough DA (1991). Leber hereditary optic neuropathy: involvement of the mitochondrial ND1 gene and evidence for an intragenic suppressor mutation. Am J Hum Genet 48(5): 935–942. https://www.ncbi.nlm.nih.gov/pubmed/?term=2018041
32. Johns DR, Neufeld MJ, and Park RD (1992). An ND-6 mitochondrial DNA mutation associated with Leber hereditary optic neuropathy. Am J Hum Genet 46(3): 428–433. http://dx.doi.org/10.1016/0006-291x(92)90479-5
33. Holt IJ, Harding AE, Petty RK, and Morgan-Hughes JA (1990). A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Biochem Biophys Res Commun 187(3): 1551–1557.https://www.ncbi.nlm.nih.gov/pubmed/?term=2137962
34. Harding AE, Holt IJ, Sweeney MG, Brockington M, and Davis MB (1992). Prenatal diagnosis of mitochondrial DNA8993 T—-G disease. Am J Hum Genet 50(3): 629–633. https://www.ncbi.nlm.nih.gov/pubmed/?term=1539598
35. Kirby DM, McFarland R, Ohtake A, Dunning C, Ryan MT, Wilson C, Ketteridge D, Turnbull DM, Thorburn DR, and Taylor RW (2004). Mutations of the mitochondrial ND1 gene as a cause of MELAS. J Med Genet 41(10): 784–789. http://dx.doi.org/10.1136/jmg.2004.020537
36. Santorelli FM, Tanji K, Kulikova R, Shanske S, Vilarinho L, Hays AP, and DiMauro S (1997). Identification of a novel mutation in the mtDNA ND5 gene associated with MELAS. Biochem Biophys Res Commun 238(2): 326–328. http://dx.doi.org/10.1006/bbrc.1997.7167
37. Corona P, Antozzi C, Carrara F, D’Incerti L, Lamantea E, Tiranti V, and Zeviani M (2001). A novel mtDNA mutation in the ND5 subunit of complex I in two MELAS patients. Ann Neurol 49(1): 106–110. http://dx.doi.org/10.1016/j.nmd.2006.05.010
39. Hanna MG, Nelson IP, Morgan-Hughes JA, and Wood NW (1998). MELAS: a new disease associated mitochondrial DNA mutation and evidence for further genetic heterogeneity. J Neurol Neurosurg Psychiatry 65(4): 512–517. http://dx.doi.org/10.1136/jnnp.65.4.512
40. Goto Y, Nonaka I, and Horai S (1990). A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 348(6302): 651–653. http://dx.doi.org/10.1038/348651a0
41. Sato W, Hayasaka K, Shoji Y, Takahashi T, Takada G, Saito M, Fukawa O, and Wachi E (1994). A mitochondrial tRNA(Leu)(UUR) mutation at 3,256 associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS). Biochem Mol Biol Int 33(6): 1055–1061. https://www.ncbi.nlm.nih.gov/pubmed/?term=7804130
42. Moraes CT, Ciacci F, Bonilla E, Jansen C, Hirano M, Rao N, Lovelace RE, Rowland LP, Schon EA, and DiMauro S (1993). Two novel pathogenic mitochondrial DNA mutations affecting organelle number and protein synthesis. Is the tRNA(Leu(UUR)) gene an etiologic hot spot? J Clin Invest 92(6): 2906–2915. http://dx.doi.org/10.1172/JCI116913
43. Hayashi J, Ohta S, Takai D, Miyabayashi S, Sakuta R, Goto Y, and Nonaka I (1993). Accumulation of mtDNA with a mutation at position 3271 in tRNA(Leu)(UUR) gene introduced from a MELAS patient to HeLa cells lacking mtDNA results in progressive inhibition of mitochondrial respiratory function. Biochem Biophys Res Commun 197(3): 1049–1055. http://dx.doi.org/10.1006/bbrc.1993.2584
44. Sakuta R, Goto Y, Horai S, and Nonaka I (1993). Mitochondrial DNA mutations at nucleotide positions 3243 and 3271 in mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes: a comparative study. J Neurol Sci 115(2): 158–160. http://dx.doi.org/10.1016/0022-510x(93)90219-o
45. Goto Y, Nonaka I, and Horai S (1991). A new mtDNA mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS). Biochim Biophys Acta 1097(3): 238–240. http://dx.doi.org/10.1016/0925-4439(91)90042-8
46. Goto YI, Tsugane K, Tanabe Y, Nonaka I, and Horai S (1994). A New Point Mutation at Nucleotide Pair-3291 of the Mitochondrial Transfer-RNALeu(Uur) Gene in a Patient with Mitochondrial Myopathy, Encephalopathy, Lactic-Acidosis, and Stroke-Like Episodes (MELAS). Biochem Biophys Res Commun 202(3): 1624–1630. http://dx.doi.org/10.1006/bbrc.1994.2119
47. Bataillard M, Chatzoglou E, Rumbach L, Sternberg D, Tournade A, Laforet P, Jardel C, Maisonobe T, and Lombes A (2001). Atypical MELAS syndrome associated with a new mitochondrial tRNA glutamine point mutation. Neurology 56(3): 405–407. http://dx.doi.org/10.1212/WNL.56.3.405
48. Shoffner JM, Lott MT, Lezza AM, Seibel P, Ballinger SW, and Wallace DC (1990). Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell 61(6): 931–937. http://dx.doi.org/10.1016/0092-8674(90)90059-n
49. Wallace DC, Zheng XX, Lott MT, Shoffner JM, Hodge JA, Kelley RI, Epstein CM, and Hopkins LC (1988). Familial mitochondrial encephalomyopathy (MERRF): genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell 55(4): 601–610. http://dx.doi.org/10.1016/0092-8674(88)90218-8
50. Masucci JP, Davidson M, Koga Y, Schon EA, and King MP (1995). In vitro analysis of mutations causing myoclonus epilepsy with ragged-red fibers in the mitochondrial tRNA(Lys)gene: two genotypes produce similar phenotypes. Mol Cell Biol 15(5): 2872–2881. http://dx.doi.org/10.1128/MCB.15.5.2872
51. Zeviani M, Muntoni F, Savarese N, Serra G, Tiranti V, Carrara F, Mariotti C, and DiDonato S (1993). A MERRF/MELAS overlap syndrome associated with a new point mutation in the mitochondrial DNA tRNA(Lys) gene. EurJ Hum Genet 1(1): 80–87. http://dx.doi.org/10.1159/000472390
52. Silvestri G, Moraes CT, Shanske S, Oh SJ, and DiMauro S (1992). A new mtDNA mutation in the tRNA(Lys) gene associated with myoclonic epilepsy and ragged-red fibers (MERRF). Am J Hum Genet 51(6): 1213–1217. https://www.ncbi.nlm.nih.gov/pubmed/?term=1361099
53. Ozawa M, Nishino I, Horai S, Nonaka I, and Goto Y-I (1997). Myoclonus epilepsy associated with ragged-red fibers: A G-to-A mutation at nucleotide pair 8363 in mitochondrial tRNALys in two families. Muscle Nerve 20(3): 271–278. http://dx.doi.org/10.1002/humu.1380030311
55. Sue CM, Tanji K, Hadjigeorgiou G, Andreu AL, Nishino I, Krishna S, Bruno C, Hirano M, Shanske S, Bonilla E, Fischel-Ghodsian N, DiMauro S, and Friedman R (1999). Maternally inherited hearing loss in a large kindred with a novel T7511C mutation in the mitochondrial DNA tRNA(Ser(UCN)) gene. Neurology 52(9): 1905–1908. http://dx.doi.org/10.1212/WNL.52.9.1905
56. Hema Bindu L, and Reddy PP (2009). Genetics of aminoglycocide-induced and prelingual non-syndromic mitochondrial hearing impairment: A review. Int J Audiol 47(11): 702–707. http://dx.doi.org/10.1080/14992020802215862
57. Fischel-Ghodsian N, Prezant TR, Bu X, and Öztas S (1993). Mitochondrial ribosomal RNA gene mutation in a patient with sporadic aminoglycoside ototoxicity. Am J Otolaryngol 14(6): 399–403. http://dx.doi.org/10.1016/0196-0709(93)90113-L
58. Hutchin T, Haworth I, Higashi K, Fischel-Ghodsian N, Stoneking M, Saha N, Arnos C, and Cortopassi G (1993). A molecular basis for human hypersensitivity to aminoglycoside antibiotics. Nucleic Acids Research 21(18): 4174–4179. https://www.ncbi.nlm.nih.gov/pubmed/?term=8414970
59. Prezant TR, Agapian JV, Bohlman MC, Bu X, Oztas S, Qiu WQ, Arnos KS, Cortopassi GA, Jaber L, and Rotter JI (1993). Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet 4(3): 289–294. http://dx.doi.org/10.1038/ng0793-289
60. Lott MT, Leipzig JN, Derbeneva O, Xie HM, Chalkia D, Sarmady M, Procaccio V, and Wallace DC (2013). mtDNA Variation and Analysis Using Mitomap and Mitomaster. Curr Protoc Bioinformatics 44(1): 1.23.1–26. http://dx.doi.org/10.1002/0471250953.bi0123s44