Back to article: Biomechanical stress provides a second hit in the establishment of BMP/TGFβ-related vascular disorders
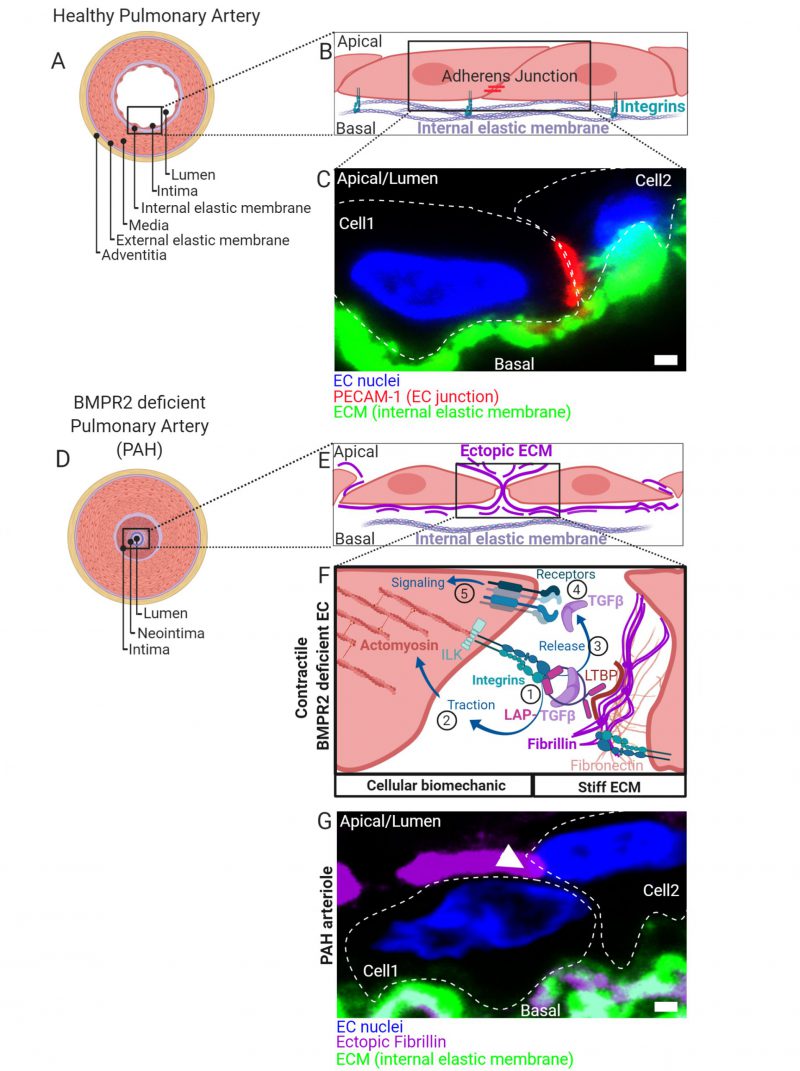
FIGURE 1: (A) Healthy pulmonary artery is characterized by a discrete macroscopic organization of tissue and extracellular matrix (ECM) layers (blue) from the inside to the outside of a blood vessel. Facing the lumen, the intima is formed by a thin layer of endothelial cells followed by a discrete ECM layer composing the internal elastic membrane. A big contribution to wall stability is made by the media formed by multiple layers of smooth muscle cells. In small capillaries such as arterioles, the wall thickness is reduced by lesser number of SMC layers. The media is connected to a second discrete layer of ECM composing the external elastic membrane facing an outer layer of cells and ECM referred to as adventitia. (B) ECs facing the lumen with their apical side connect to neighboring cells through distinct contact sites and to the internal elastic membranes by integrin-rich focal adhesions. Complexes of integrins are composed of α/β-subunits. Shown are adherens- junctions (red) at contact sites and integrin complexes engaging with ECM proteins of the basal lamina of the internal elastic membrane. (C) Immunohistochemical fluorescence staining of transversal cross-sectioned human pulmonary arterioles. The region of interest shown depicts on two adjacent EC nuclei (blue) from cells constituting the intima with junctional marker protein platelet endothelial cell adhesion molecule-1 (PECAM-1) shown in red together with the internal elastic membrane in green. Cell body boundaries as slash dotted lines. (D) BMP receptor type II (BMPR2) deficiency primes for pulmonary arterial hypertension (PAH). During PAH, the discrete macroscopic and microscopic organization of the tissue layers lining the blood vessel lumen is disturbed. Histologically most pronounced and dependent on the disease stage, the media thickens by hypertrophy of SMCs. Moreover, internal and external elastic membranes remodel, external cells invade and excessive ECM deposits are found ectopically. The lumen is obstructed and a neointima is formed. At later stages, vascular lesions can form (not shown) where discrete tissue borders disappear. The molecular details and the contribution of intimal thickening and neointima formation are less defined. Trans-differentiation of ECs towards a myofibroblast-like cell type is suggested. (E) We found that BMPR2-deficient ECs deposit ectopic ECM in their cell-junctions (fibrillin) and that the general composition of the ECM (Matrisome) changes. (F) In the absence of BMPR2, ECs form active mechano-complexes containing β1-integrins and Integrin-linked kinase (ILK) at cell contact sites on the expense of VE-Cadherin and PECAM-1 localization. The ectopic ECM is decorated with the latency associated peptide (LAP) bound TGFβ. (1) In BMPR2-deficient cells β1-integrin-ILK containing mechanocomplexes bind to a RGD-motive within the latency associated peptide (LAP) of TGFβ. Latent TGFβ binding to the ECM is facilitated by increased formation of fibronectin fibers acting as a template for fibrillin fibrils to which the latent-transforming growth factor beta-binding protein (LTBP) facilitates the LAP (TGFβ) tethering. In its ECM-bound form, this latency complex renders TGFβ to be biologically not accessible for cells. (2) Intracellular traction forces are generated by RHO kinase dependent actomyosin contractility. The contractile actomyosin cytoskeleton connects to integrins via the adaptor protein ILK. (3) Mature TGFβ is released from LAP by integrin binding and actomyosin contraction. Conformational changes of the latency complex expose protease cleavage sites allowing for the retrieval of soluble mature TGFβ. ECM stiffness is pivotal to this process providing resistance to the cell-generated traction. (4) Mature TGFβ is able to bind to BMP/TGFβ receptor complexes comprising high affinity TGFβ receptor type II. Activated receptor complexes induce intracellular signalling cascades of which we have studied SMAD-dependent signaling in detail in Hiepen C, Jatzlau J. et al. PLoS Biol 2019. The mechanical aspects of this mechanism is suppressed in ECs, when BMPR2 is present, making it an important gatekeeper molecule. (G) Immunohistochemical fluorescence staining of transversal cross-sectioned pulmonary arterioles from a PAH donor. The region of interest shown depicts on two adjacent EC nuclei (blue) from the neointima with ectopic fibrillin deposits at the basal and apical side and the internal elastic membrane shown in green. Ectopic fibrillin deposits at cell contact sites, white arrow head. Cell body boundaries, slash dotted lines. Scale bar is 2µ#x03BC;m.