Back to article: Rethinking the bioavailability and cellular transport properties of S-adenosylmethionine
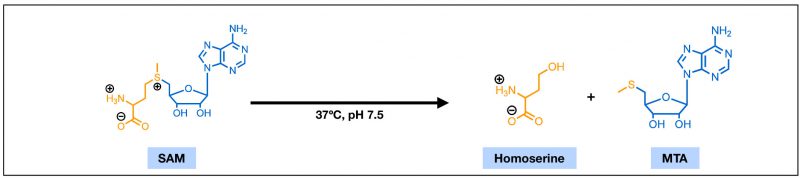
FIGURE 1: The chemical structures of S-adenosylmethionine (SAM) and 5'-methylthioadenosine (MTA). With features of an amino acid (yellow) and a ribose nucleotide (blue), SAM is highly polar. Under physiological conditions, SAM can undergo a non-enzymatic cleavage reaction and degrade into homoserine and MTA.