News and thoughts:
Cell Stress, Vol. 6, No. 8, pp. 72 - 75; doi: 10.15698/cst2022.08.270
Cardiac PI3K p110α attenuation delays aging and extends lifespan
1Department of Cardiology, Medical University of Graz, 8036 Graz, Austria.
2Centre de Recherche des Cordeliers, Equipe labellisée par la Ligue contre le cancer, Université de Paris, Sorbonne Université, Inserm U1138, Institut Universitaire de France, Paris 75006, France.
3Metabolomics and Cell Biology Platforms, Institut Gustave Roussy, Villejuif 94805, France.
4BioTechMed Graz, 8010 Graz, Austria.
5Institute of Molecular Biosciences, NAWI Graz, University of Graz, 8010 Graz, Austria.
6Field of Excellence BioHealth, University of Graz, 8010 Graz, Austria.
7Institute of Biochemistry and Center for Molecular Biosciences Innsbruck, University of Innsbruck, Innsbruck 6020, Austria.
8Department of Pediatrics, Section Systems Medicine of Metabolism and Signalingg, University of Groningen, University Medical Center Groningen, Groningen 9700 RB, The Netherlands.
9Department for Neuroscience, School of Medicine and Health Sciences, Carl von Ossietzky University Oldenburg, Oldenburg 26129, Germany.
10Institut du Cancer Paris CARPEM, Department of Biology, Hôpital Européen Georges Pompidou, AP-HP, Paris 7015, France.
11Institute of Physiology, Faculty of Medicine, University of Maribor, 2000 Maribor, Slovenia.
Keywords: PI3K, IGF1, insulin signaling, cardiomyopathy, heart failure, aging, autophagy, mitochondrial dysfunction.
Received originally: 25/07/2022 Accepted: 28/07/2022
Published: 08/08/2022
Correspondence:
Mahmoud Abdellatif, Department of Cardiology, Medical University of Graz, Auenbruggerplatz 15, A-8036 Graz, Austria; mahmoud.abdellatif@medunigraz.at
Simon Sedej, Department of Cardiology, Medical University of Graz, Auenbruggerplatz 15, A-8036 Graz, Austria; simon.sedej@medunigraz.at
Conflict of interest statement:
G.K. has been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Sotio, Tollys, Vascage and Vasculox/Tioma. G.K. has been consulting for Reithera. G.K. is on the Board of Directors of the Bristol Myers Squibb Foundation France. G.K. is a scientific cofounder of everImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio. G.K. is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis and metabolic disorders. The other authors have nothing to declare. T.E. has equity interests and is an advisor for The Longevity Labs (TLL). T.E. is also an inventor of patents covering the use of spermidine for health benefits. M.A. and S.S. are involved in a patent application dealing with the cardiometabolic effects of caloric restriction mimetics, like spermidine and nicotinamide.
Please cite this article as: Abdellatif et al. (2022). Cardiac PI3K p110α attenuation delays aging and extends lifespan. Cell Stress: 6(8): 72-75. doi: 10.15698/cst2022.08.270
Phosphoinositide 3-kinase (PI3K) is a key component of the insulin signaling pathway that controls cellular metabolism and growth. Loss-of-function mutations in PI3K signaling and other downstream effectors of the insulin signaling pathway extend the lifespan of various model organisms. However, the pro-longevity effect appears to be sex-specific and young mice with reduced PI3K signaling have increased risk of cardiac disease. Hence, it remains elusive as to whether PI3K inhibition is a valid strategy to delay aging and extend healthspan in humans. We recently demonstrated that reduced PI3K activity in cardiomyocytes delays cardiac growth, causing subnormal contractility and cardiopulmonary functional capacity, as well as increased risk of mortality at young age. In stark contrast, in aged mice, experimental attenuation of PI3K signaling reduced the age-dependent decline in cardiac function and extended maximal lifespan, suggesting a biphasic effect of PI3K on cardiac health and survival. The cardiac anti-aging effects of reduced PI3K activity coincided with enhanced oxidative phosphorylation and required increased autophagic flux. In humans, explanted failing hearts showed increased PI3K signaling, as indicated by increased phosphorylation of the serine/threonine-protein kinase AKT. Hence, late-life cardiac-specific targeting of PI3K might have a therapeutic potential in cardiac aging and related diseases.
–
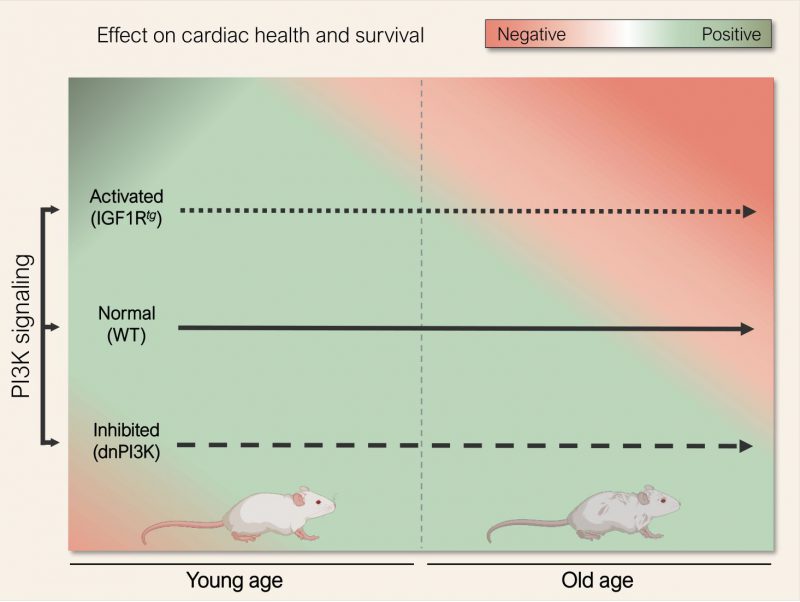
Life expectancy continues to rise worldwide, and so does the prevalence and socioeconomic burden of age-related chronic diseases. Amongst these, cardiovascular disorders remain the leading cause of lost healthy life years and ensuing mortality. Thus, defining the molecular and cellular mechanisms dictating the pace of cardiac aging is required for the development of novel interventions that might improve outcomes in cardiovascular disease, beyond the avoidance of traditional risk factors. The inhibition of insulin/insulin-like growth factor-1 signaling (IIS) pathway is an evolutionary conserved mechanism to delay organismal aging. Loss-of-function mutations in key components of the IIS pathway extend the lifespan in various organisms, ranging from yeast to rodents [1][2]. However, the magnitude of such anti-aging effect appears to depend on sex and the genetic background of tested animals [3]. Since IIS is required for normal growth and metabolism, further studies – on druggable targets of the IIS pathway and preferably in a cell type-specific fashion – must determine whether inhibiting IIS pathway is a valid and safe strategy to compress late-life morbidity in humans. In this respect, aged mice with reduced cardiac activity of phosphoinositide 3-kinase (PI3K), a key downstream effector of the IIS pathway, exhibit preserved cardiac function and reduced expression of senescence markers [4]. By contrast, young mice with cardiomyocyte-specific suppression of PI3K are more prone to dilated and ischemic cardiomyopathy, at least when surgically induced at a young age [5][6]. In order to resolve these disparate findings, we performed a comprehensive long-term study, in which we assessed cardiac health and survival of two transgenic mouse models with increased or reduced cardiac PI3K signaling throughout the course of life [7].
We observed that mice harbouring an inactive PI3K p110α isoform specifically in cardiac myocytes (dnPI3K) show delayed cardiac growth, subnormal contractility, and attenuated cardiopulmonary functional capacity, leading to an abnormally increased risk of mortality during early life. In contrast, aged dnPI3K mice displayed an attenuated age-dependent decline in cardiac systolic and diastolic functions, preserved cardiac functional reserve, reduced cardiac remodelling as well as improved myocardial bioenergetics, which were associated with extended survival during late-life stages. Mechanistically, delayed cardiac aging in dnPI3K mice coincided with activated autophagic flux in the heart. In fact, autophagy inhibition using the lysosomotropic agent hydroxychloroquine abolished most of the benefits observed in aged dnPI3K hearts, indicating that functional autophagy has a causal role in the late-life cardioprotective effects of reduced PI3K activity.
Conversely, increased IIS-PI3K signaling in mice overexpressing the human insulin-like growth factor-1 in cardiac myocytes (IGF1Rtg) led to augmented heart growth, supranormal contractile function and increased exercise capacity. However, aged IGF1Rtg mice developed signs of accelerated cardiac aging, as indicated by reduced contractility and exacerbated diastolic dysfunction, leading to effort intolerance, compromised cardiopulmonary functional capacity, lung congestion and shorter lifespan. Aged IGF1Rtg mice also exhibited increased left ventricular fibrosis as well as severe left atrial remodelling, suggesting an age-related shift from physiological hypertrophy towards hypertrophic cardiomyopathy. Premature cardiac aging in IGF1Rtg mice correlated with mitochondrial dysfunction and reduced anti-oxidative potential, likely due to blocked cardiac autophagy. In support of this notion, reduced autophagic flux occurred before the development of these deleterious cardiac effects, while reactivating autophagy by the caloric restriction mimetic spermidine prevented the deterioration of oxidative phosphorylation and stress resistance mechanisms, thereby preserving cardiac function and protecting aged IGF1Rtg mice from heart failure [8].
Our findings are likely clinically relevant because we observed cardiac IGF1R overexpression and increased phosphorylation of the serine/threonine-protein kinase AKT in left ventricular samples of patients with non-ischemic dilated cardiomyopathy, denoting activated cardiac IIS-PI3K signaling in heart failure. Downstream to PI3K, we detected increased mammalian target of rapamycin (MTOR)-dependent inhibitory phosphorylation of unc-51 like autophagy activating kinase 1 (ULK1) [9]. Intriguingly, and unlike failing human hearts, donors with compensated hypertrophy (i.e., preserved function, despite increased remodelling) showed no signs of increased IIS-PI3K signaling as compared to age-matched controls with no history of cardiac disease, suggesting a potential protective role of IIS-PI3K inhibition.
In sum, age appears to be a key determining factor that defines the role of PI3K signaling in the heart. At young age, increased activity of the IIS-PI3K axis is required for normal cardiac growth and development. However, later in life reduced PI3K activity confers anti-aging effects, likely through prioritizing cardioprotective quality control mechanisms, such as autophagy, over superfluous cardiac growth (Fig. 1). Such a biphasic relationship between cardiac health and IIS-PI3K signaling activity reconciles previous opposing studies [4][6], and suggests that partial inhibition of PI3K might be worth considering for the treatment of heart failure and other age-related chronic diseases – albeit at a much lower and safer dosage than that applied in cancer therapy [10][11]. Alternatively, emerging autophagy inducers and caloric restriction mimetics, like spermidine and nicotinamide adenine dinucleotide (NAD+) precursors [8][12][13][14], might offer a readily-available, and perhaps safer, strategy for immediate testing in cardiac patients [15].
–
REFERENCES
- Foukas LC, Bilanges B, Bettedi L, Pearce W, Ali K, Sancho S, Withers DJ, and Vanhaesebroeck B (2013). Long-term p110α PI3K inactivation exerts a beneficial effect on metabolism. EMBO Mol Med 5(4): 563–571. 10.1002/emmm.201201953
- Milman S, Huffman DM, and Barzilai N (2016). The Somatotropic Axis in Human Aging: Framework for the Current State of Knowledge and Future Research. Cell Metab 23(6): 980–989.. 10.1016/j.cmet.2016.05.014
- Sell C (2015). Minireview: The Complexities of IGF/Insulin Signalingg in Aging: Why Flies and Worms Are Not Humans. Mol Endocrinol 29(8): 1107–1113. 10.1210/me.2015-1074
- Inuzuka Y, Okuda J, Kawashima T, Kato T, Niizuma S, Tamaki Y, Iwanaga Y, Yoshida Y, Kosugi R, Watanabe-Maeda K, Machida Y, Tsuji S, Aburatani H, Izumi T, Kita T, and Shioi T (2009). Suppression of phosphoinositide 3-kinase prevents cardiac aging in mice. Circulation 120(17): 1695–1703. 10.1161/CIRCULATIONAHA.109.871137
- McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, and Izumo S (2003). Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A 100(21): 12355–12360. 10.1073/pnas.1934654100
- Lin RCY, Weeks KL, Gao X-M, Williams RBH, Bernardo BC, Kiriazis H, Matthews VB, Woodcock EA, Bouwman RD, Mollica JP, Speirs HJ, Dawes IW, Daly RJ, Shioi T, Izumo S, Febbraio MA, Du X-J, and McMullen JR (2010). PI3K(p110 alpha) protects against myocardial infarction-induced heart failure: identification of PI3K-regulated miRNA and mRNA. Arterioscler Thromb Vasc Biol 30(4): 724–732. 10.1161/ATVBAHA.109.201988
- Abdellatif M, Trummer-Herbst V, Heberle AM, Humnig A, Pendl T, Durand S, Cerrato G, Hofer SJ, Islam M, Voglhuber J, Ramos Pittol JM, Kepp O, Hoefler G, Schmidt A, Rainer PP, Scherr D, von Lewinski D, Bisping E, McMullen JR, Diwan A, Eisenberg T, Madeo F, Thedieck K, Kroemer G, and Sedej S (2022). Fine-Tuning Cardiac Insulin-Like Growth Factor 1 Receptor Signalingg to Promote Health and Longevity. Circulation 145(25): 1853–1866. 10.1161/CIRCULATIONAHA.122.059863
- Abdellatif M, Madeo F, Kroemer G, and Sedej S (2022). Spermidine overrides INSR (insulin receptor)-IGF1R (insulin-like growth factor 1 receptor)-mediated inhibition of autophagy in the aging heart. Autophagy 1–3. 10.1080/15548627.2022.2095835
- Zimmermann A, Madreiter-Sokolowski C, Stryeck S, and Abdellatif M (2021). Targeting the Mitochondria-Proteostasis Axis to Delay Aging. Front Cell Dev Biol 9: 656201. 10.3389/fcell.2021.656201
- Nunnery SE, and Mayer IA (2019). Management of toxicity to isoform α-specific PI3K inhibitors. Annals of Oncology 30: x21–x26. 10.1093/annonc/mdz440
- Sala V, Della Sala A, Ghigo A, and Hirsch E (2021). Roles of phosphatidyl inositol 3 kinase gamma (PI3Kγ) in respiratory diseases. Cell Stress 5(4): 40–51. 10.15698/cst2021.04.246
- Abdellatif M, Sedej S, and Kroemer G (2021). NAD+ Metabolism in Cardiac Health, Aging, and Disease. Circulation 144(22): 1795–1817. 10.1161/CIRCULATIONAHA.121.056589
- Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, Harger A, Schipke J, Zimmermann A, Schmidt A, Tong M, Ruckenstuhl C, Dammbrueck C, Gross AS, Herbst V, Magnes C, Trausinger G, Narath S, Meinitzer A, Hu Z, Kirsch A, Eller K, Carmona-Gutierrez D, Büttner S, Pietrocola F, Knittelfelder O, Schrepfer E, Rockenfeller P, Simonini C, Rahn A, Horsch M, Moreth K, Beckers J, Fuchs H, Gailus-Durner V, Neff F, Janik D, Rathkolb B, Rozman J, de Angelis MH, Moustafa T, Haemmerle G, Mayr M, Willeit P, von Frieling-Salewsky M, Pieske B, Scorrano L, Pieber T, Pechlaner R, Willeit J, Sigrist SJ, Linke WA, Mühlfeld C, Sadoshima J, Dengjel J, Kiechl S, Kroemer G, Sedej S, and Madeo F (2016). Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med 22(12): 1428–1438. 10.1038/nm.4222
- Abdellatif M, Trummer-Herbst V, Koser F, Durand S, Adão R, Vasques-Nóvoa F, Freundt JK, Voglhuber J, Pricolo M-R, Kasa M, Türk C, Aprahamian F, Herrero-Galán E, Hofer SJ, Pendl T, Rech L, Kargl J, Anto-Michel N, Ljubojevic-Holzer S, Schipke J, Brandenberger C, Auer M, Schreiber R, Koyani CN, Heinemann A, Zirlik A, Schmidt A, von Lewinski D, Scherr D, Rainer PP, von Maltzahn J, Mühlfeld C, Krüger M, Frank S, Madeo F, Eisenberg T, Prokesch A, Leite-Moreira AF, Lourenço AP, Alegre-Cebollada J, Kiechl S, Linke WA, Kroemer G, and Sedej S (2021). Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci Transl Med 13(580): eabd7064. 10.1126/scitranslmed.abd7064
- Sedej S, and Abdellatif M (2022). Metabolic therapy for managing heart failure with preserved ejection fraction. J Mol Cell Cardiol 168: 68-69. 10.1016/j.yjmcc.2022.04.009
ACKNOWLEDGMENTS
This work was funded by the Austrian Science Fund (FWF) through grants P27637-B28 and I3301-MINOTAUR to S.S.. M.A. acknowledges support from the Austrian Society of Cardiology (Präsidentenstipendium-ÖKG), Medical University of Graz (Start Fund) and the European Commission (H2020-MSCA-IF, Nr. 101025118). G.K. is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Association “Ruban Rose”; Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno- Screen; European Joint Programme on Rare Diseases (EJPRD); Gustave Roussy Odyssea, the European Union Horizon 2020 Projects Oncobiome and Crimson; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085), Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001); the Leducq Foundation; the RHU Torino Lumière; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX- 0001. K.T. acknowledges support from the MESI-STRAT project (Grant Agreement 754688), PoLiMeR Innovative Training Network (Marie Skłodowska-Curie Grant Agreement 812616 ), the ARDRE programme (Marie Skłodowska-Curie grant agreement No 847681), which all received funding from the European Union Horizon 2020 Research and Innovation Program, and the PARC partnership which has received funding from the European Union's Horizon Europe Research and Innovation Programme under Grant Agreement No 101057014. A.H. is supported by the Tyrolean Science Fund (TWF; grant agreement F.33468/7–2021).
COPYRIGHT
© 2022

Cardiac PI3K p110α attenuation delays aging and extends lifespan by Abdellatif et al is licensed under a Creative Commons Attribution 4.0 International License.