Back to article: VDAC1 at the crossroads of cell metabolism, apoptosis and cell stress
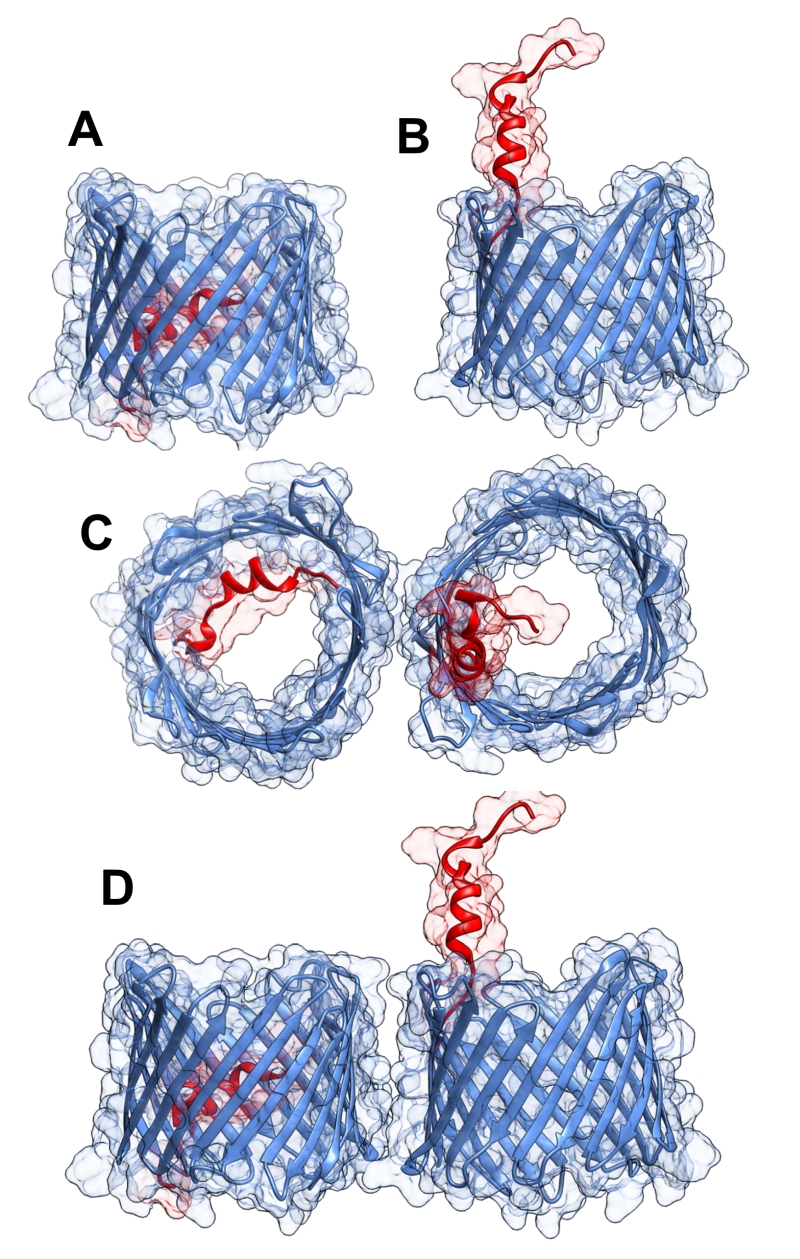
FIGURE 1: Three-dimensional structure of VDAC1. VDAC1 monomer and dimer structures. (A) Side-view of the crystal structure of VDAC1 (PDB code: 3EMN). The β-barrel is formed by 19 β strands and the N-terminal domain (colored red) is folded into the pore interior. (B) A proposed model for the conformation of VDAC1 with its N-terminal on the outside of the VDAC1 pore. (C) Top-view of VDAC1 dimer with the N-terminal helix nested inside the VDAC1 pore in one monomer and outside of the pore in the other. (D) Side-view of proposed dimer of VDAC1. Figures were prepared using PyMOL software.