News and thoughts:
Cell Stress, Vol. 7, No. 10, pp. 90 - 94; doi: doi: 10.15698/cst2023.10.290
Gut microbiota turn up the heat after bariatric surgery
1 Department of General, Visceral, Transplantation, Vascular and Pediatric Surgery, University Hospital Wuerzburg, Wuerzburg, Germany.
Keywords: vertical sleeve gastrectomy, Roux-en-Y gastric bypass, gut microbiota, thermogenesis, obesity, Type 2 diabetes.
Received originally: 03/08/2023 Accepted: 13/08/2023
Published: 15/08/2023
Correspondence:
Mohammed K. Hankir, Center of Operative Medicine, Oberduerrbacherstr. 6, 97080 Wuerzburg, Germany; hankir_m@ukw.de
Conflict of interest statement: The author has no conflicts of interest to declare.
Please cite this article as: Mohammed K. Hankir (2023). Gut microbiota turn up the heat after bariatric surgery. Cell Stress 7(10): 90-94. doi: 10.15698/cst2023.10.290
Bariatric surgeries like vertical sleeve gastrectomy (VSG) and Roux-en-Y gastric bypass (RYGB) cause well-established shifts in the gut microbiota, but how this contributes to their unique metabolic benefits is poorly understood. Jin et al and Yadav et al now provide two complementary lines of evidence suggesting that gut microbiota-derived metabolites after VSG and RYGB activate thermogenesis in fat through distinct mechanisms, to in turn promote weight loss and/or improvements in glycemic control.
Despite the implementation of numerous weight loss initiatives by various governments, the global prevalence of obesity continues to rise [1]. This not only takes an often dismissed personal toll on many individuals living with obesity [2], but also represents an enormous burden to healthcare systems [3] and is a leading preventable cause of death [1]. Of the treatments that are currently available for severe obesity, bariatric surgeries such as vertical sleeve gastrectomy (VSG) and Roux-en-Y gastric bypass (RYGB) remain the most effective [4]. These surgeries not only cause unparalelled levels of weight loss (up to 25-35%) [5], but also induce a host of other metabolic benefits including remission of the type 2 diabetes [6] and amelioration of the fatty liver disease [7] that usually accompany obesity. A number of mechanisms have been proposed to contribute to these favourable metabolic outcomes, most notably the rise in circulating gut hormones like glucacon-like peptide 1 (GLP-1) [8]. There are also well-established shifts in the gut microbiota after bariatric surgery, although this appears to be more pronounced for RYGB than for VSG likely due to the differences in anatomical configurations between the two procedures [9]. Interestingly, while gut hormone preperations including the stable GLP-1 analogue semaglutide cause weight loss approaching the level of bariatric surgery (up to 10-20%) [10], GLP-1 receptor signalling seems to be dispensable for the metabolic benefits of both VSG [11] and RYGB [12], at least in rodent models. Therefore, the hunt is still very much on for the identification of factors that contribute to the weight loss and improved glycemic control associated with bariatric surgery, as this will undoubtedly aid in the discovery of novel anti-obesity and type 2 diabetes drugs.
–
Since its rediscovery in adult humans, research into thermogenic fat has increased tremendously [13]. This is because activation of thermogenesis in fat either by cold exposure or by pharmacological means has long been known to promote metabloic health in rodents, and it is becoming increasingly evident that it does so in the clinical setting, too [13]. While both gut microbiota and bariatric surgery have been shown to activate thermogenesis in fat [14], their connection has, until recently, not been addressed. Two new studies by Jin et al [15] and Yadav et al [16] now provide causal evidence, using complementary approaches, that VSG and RYGB activate thermogenesis in fat through distinct microbiota-dependent mechanisms, to in turn promote metabolic health.
–
In the first study by Jin et al [15] VSG and sham surgery were performed on diet-induced obese mice. The authors found that VSG-operated mice displayed reduced body weight associated with increased oxygen consumption compared with sham-operated mice. Interestingly, this was not associated with lasting reductions in food intake in VSG-operated mice, reiterating how rodent models of bariatric surgery achieve weight loss predominantly through increasing energy expenditure rather than through decreasing energy intake, which is the opposite to the case in humans [17]. When the authors analysed the 3 major fat depots in mice, they found that the thermogenic mitochondrial molecule uncoupling protein 1 (UCP1) was increased only in subcutaneous white fat and not in epididymal white fat or interscapular brown fat. Because the sympathetic nervous system is an important mediator of thermogenesis, the authors then denervated subcutaneous white fat by injecting the neurotoxin 6-hydroxydopamine locally into this depot. Unexpectedly, this had minimal impact on the efficacy of VSG to exert its metabolic benefits and to increase UCP1 expression in subcutaneous white fat. At this point, it should be stated that these findings contrast sharply with the impact of VSG on diet-induced obese rats, in which it was shown that sympathetic nervous system innervation of brown fat is essential for the reductions in body weight and improvements in glycemic control postoperatively [18], again reiterating species differences in the mechanisms of bariatric surgery.
–
Jin et al [15] then reasoned that gut microbiota-derived products could mediate the activation of thermogenesis in subcutaneous white fat after VSG. Indeed, the authors could confirm that VSG increased the fecal abundance of Firmicutes and decreased the fecal abundance of Bacteriodetes, associated with stabilization of the intestinal epithelial barrier. Mass spectrometry analysis then revealed that 3 metabolites were increased in the feces of VSG-operated mice: licoricidin, muramic acid, and 3-hydroxybutyryl carnitine. Moreover, fecal licoricidin not only negatively correlated with various gut microbiota known to promote metabolic disease, but circulating licoricidin levels were doubled in VSG-operated mice suggesting that it could communicate directly with subcutaneous white fat. To formally test if gut microbiota-derived products contribute to thermogenesis in subcutaneous white fat after VSG, mice were treated with a broad spectrum antibiotic cocktail via their drinking water for 2 weeks. Strikingly, this not only prevented the weight loss and metabolic benefits associated with VSG similar to a previous study in diet-induced obese mice [19], but it also prevented the increase in oxygen consumption and subcutaneous white fat UCP1 expression as well as the increase in circulating licoricidin. However, this relatively unspecific approach does not reveal which gut microbiota produce and release licoricidin, which is an isoflavenoid normally found in dietary licorice, nor does it prove that licoricidin per se contributes to the activation of thermogenesis in subcutaneous white fat after VSG.
–
Next, Jin et al [15] determined if licoricidin given alone via oral gavage can at least recapitulate the metabolic benefits of VSG. Remarkably, licoricidin when admistered via this route prevented body weight gain, improved glycemic control, increased oxygen consumption and increased UCP1 expression in subcutaneous white fat. To dissect the molecular mechanism by which licoricidin activates thermogenesis in subcutaneous white fat, cell culture experiments were performed. Like the sympathetic nervous system, licoricidin recruited the protein kinase A (PKA) signalling pathway in primary subcutaneous white adipocytes as shown through phosphoblots of PKA substrates including cyclic AMP response element binding protein (CREB). Interestingly, the tripling of intacellular cAMP levels by licoricidin treatment did not appear to be due to the inhibition of phosphodiesterases 3 and 4 which is known to promote thermogenesis in epididymal white fat [20][21] and subcutaneous white fat [22], respectively. Instead, through a combination of confocal microscopy on primary subcutaneous white adipocytes, pull-down assays on beta-3 adrenergic receptor-overexpressing human embryonic kidney (HEK) cells and bioinformatic analysis, licoricidin was shown to bind to transmembrane 3 (TM3), TM6 and TM7 of the beta-3 adrenergic receptor. Moreover, the induction of UCP1 protein in primary subcutaneous white adipocytes by licoricidin was abrogated in cells with knockdown of the beta-3 adrenergic receptor, providing strong evidence that licoricidin promotes thermogenesis in subcutaneous white fat by positively modulating beta-3 adrenergic receptor activity. Finally, to determine if local licoricidin action in subcutaneous white fat is sufficient to recapitulate the effects of systemic licoricidin, it was injected at lower doses directly into this depot. This targetted licoricidin treatment had overlapping metabolic effects with systemic licoricidin treatment, although experiments in PR domain containing 16 (PRDM16)-deficient mice, which are incapable of showing themogenesis in subcutaneous white fat in response to beta-3 adrenergic receptor agonist treatment [23], are needed to determine to what extent activation of thermogenesis in subcutaneous white fat by licoricidin contributes to the metabolic benefits of VSG as well as systemic licoricidin treatment.
–
In the other study by Yadav et al [16] stool samples were collected from 4 patients with morbid obesity before and 1-6 months after RYGB, and subsequently transferred to germ-free mice. What sets the approach of Yadav et al [16] apart from similar studies is that samples before and after RYGB from the same individual patient were orally gavaged into recipient germ-free mice in a longitudinal design rather than in a cross-sectional design, which preserves the inter-individual variability in the response to bariatric sugery. Another notable detail of the study of Yadav et al [16] is that the germ-free mice in their study were rendered obese on a sterile and costly Western-style diet prior to receiving stool samples from patients. Perhaps equally as important is the delayed timepoint after fecal microbiota transfer (12 weeks) that recipient germ-free mice were subjected to metabolic phenotyping, allowing sufficient time for the transferred fecal microbiota to expand and colonize the gastrointestinal tract and exert metabolic effects. In this way, Yadav et al [16] found that mice receiving post-RYGB feces have improved glycemic control and increased oxygen consumption compared to mice receiving pre-RYGB feces. Notably, this was independent of any effects on food intake and body weight, suggesting that post-RYGB fecal microbiota have little impact on regulating energy homeostasis. The authors then performed 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) imaging experiments to determine if brown fat contributes to increased energy expenditure in mice receiving post-RYGB fecal microbiota. This revealed that brown fat 18F-FDG uptake in response to overnight cold exposure, the natural stimulus for brown fat, almost doubles in mice receiving post-RYGB feces compared with mice receiving pre-RYGB feces. Accordingly, brown fat UCP1 protein expression was also markedly enhanced.
–
Considering that the post-RYGB feces-treated mice showed enhanced insulin sensitivity in the absence of any differences in body weight, it would have been interesting to determine if insulin increases brown fat 18F-FDG uptake. This could have provided evidence that post-RYGB microbiota enhance blood glucose clearance in response to insulin treatment through using brown fat as a glucose sink. It would have also been interesting to determine if the patients themselves showed increased oxygen consumption and brown fat 18F-FDG uptake after RYGB, which would have provided the key evidence that this beneficial metabolic change in the host is potentially gut microbiota-mediated and is transmissable to germ-free mice.
–
Finally, after showing that differences in patient fecal microbiota after RYGB can be transferred to germ-free mice, such as an increase in Akkermansia municiphila, Yadav et al performed metabolomic analyses of their feces. This revealed that thermogenic molecules like the short chain fatty acid butyrate [24][25] and acylcarnitine [26] were increased in mice receiving post-RYGB fecal microbiota along with tryptophan metabolites, while various amino acids including the branched chain ones valine, leucine and isoleucine which are thought to induce insulin resistance [27] were reduced although this has been shown not to be essential for the improvements in glycemic control after bariatric surgery [28]. Considering that for brown fat to be affected by these metabolites they need to first reach the circulation, it would have been interesting to perform metabolomics on systemic blood as well as feces like in the study of Jin et al [15]. It would have also been interesting to determine if butyrate does indeed contribute to activation of brown fat themogenesis and improved glycemic control by post-RYGB fecal microbiota through inhibiting monocaroxylate transporter 1 (MCT1) which has been shown to mediate butyrate's thermogenic actions on brown adipocytes [25].
–
While we are witnessing the dawn of a new era for obesity pharmacotherapy with gut hormone preperations causing unprecendented levels of weight loss, bariatric surgery remains the most effective [4]. The high quality studies of Jin et al [15] and Yadav et al [16] are groundbreaking in the field as they provide causal evidence that the well-established shifts in gut microbiota after bariatric surgery contribute to improved metablic outcomes by activating thermogenesis in fat, possibly through microbial metabolites such as licoricidin and butyrate (Figure 1). Notably, changes in bile acid metabolism by gut microbiota after VSG leads to the generation of cholic acid-7-sulfate in the liver and its accumulation in the gut [29], which has been shown to promote metabolic health in diet-induced obese mice through increasing endogenous GLP-1 release [30]. Thus, understanding the molecular and cellular mechanisms behind how bariatric surgery favourably impacts various metabolic tissues provides a treasure trove for identifying novel factors to treat obesity and type 2 diabetes.
–
The findings of Jin et al [15] and Yadav et al [16] demonstrate that the shifts in gut microbiota after RYGB and VSG lead to increased gut levels of butyrate and licoricidin, respectively. This then leads to activation of brown fat thermogenesis after RYGB, possibly via a mechanism involving monocarboxlate transporter 1 (MCT1)-mediated transport of circulating butyrate into brown adipocytes [25], and activation of subcutaneous white fat thermogenesis after VSG, possibly via a mechanism involving the positive modulation of beta-3 adrenergic receptor in white adipocytes by increased circulating licoricidin [15].
–
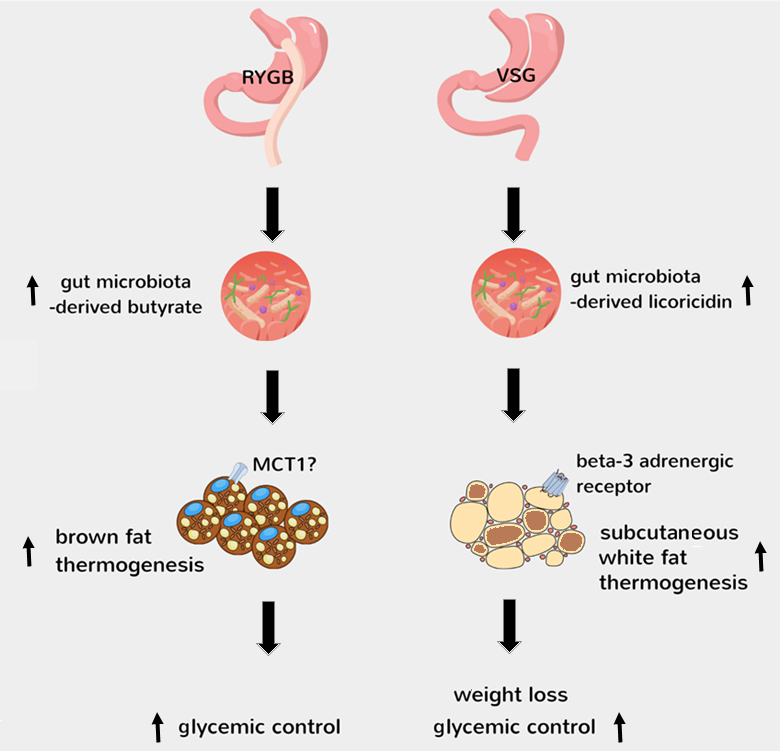 |
FIGURE 1: Mechanisms behind activation of thermogenesis by gut microbiota after bariatric surgery. The findings of Jin et al [15] and Yadav et al [16] demonstrate that the shifts in gut microbiota after RYGB and VSG lead to increased gut levels of butyrate and licoricidin, respectively. This then leads to activation of brown fat thermogenesis after RYGB, possibly via a mechanism involving monocarboxlate transporter 1 (MCT1)-mediated transport of circulating butyrate into brown adipocytes [25], and activation of subcutaneous white fat thermogenesis after VSG, possibly via a mechanism involving the positive modulation of beta-3 adrenergic receptor in white adipocytes by increased circulating licoricidin [15]. |
REFERENCES
- Afshin, A., et al. (2017). Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med 377(1):13-27. 10.1056/NEJMoa1614362
- Puhl RM, King KM (2013). Weight discrimination and bullying. Best Pract Res Clin Endocrinol Metab 27(2):117-27. 10.1016/j.beem.2012.12.002
- Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M (2011). Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378(9793):815-25. 10.1016/S0140-6736(11)60814-3
- Müller, TD, Blüher, M, Tschöp, MH, RD DiMarchi (2022). Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov 21(3):201-223. 10.1038/s41573-021-00337-8
- Adams TD, Davidson LE, Litwin SE, Kim J, Kolotkin RL, Nanjee MN, Gutierrez JM, Frogley SJ, Ibele AR, Brinton EA, Hopkins PN, McKinlay R, Simper SC, Hunt SC (2017). Weight and Metabolic Outcomes 12 Years after Gastric Bypass. N Engl J Med 377(12):1143-1155. 10.1056/NEJMoa1700459
- De Luca M, Zese M, Bandini G, Chiappetta S, Iossa A, Merola G, Piatto G, Tolone S, Vitiello A, Silverii GA, Ragghianti B, Mannucci E, Monami M; Expert Panel and Evidence Review Team for the Italian Guidelines on Bariatric and Metabolic Surgery (2023). Metabolic bariatric surgery as a therapeutic option for patients with type 2 diabetes: A meta-analysis and network meta-analysis of randomized controlled trials. Diabetes Obes Metab 25(8):2362-2373. 10.1111/dom.15117
- Lassailly G, Caiazzo R, Ntandja-Wandji LC, Gnemmi V, Baud G, Verkindt H, Ningarhari M, Louvet A, Leteurtre E, Raverdy V, Dharancy S, Pattou F, Mathurin P (2020). Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology 159(4):1290-1301.e5. 10.1053/j.gastro.2020.06.006
- Yin M, Wang Y, Han M, Liang R, Li S, Wang G, Gang X (2023). Mechanisms of bariatric surgery for weight loss and diabetes remission. J Diabetes. 10.1111/1753-0407.13443
- Coimbra VOR, Crovesy L, Ribeiro-Alves M, Faller ALK, Mattos F, Rosado EL (2022). Gut Microbiota Profile in Adults Undergoing Bariatric Surgery: A Systematic Review. Nutrients 14(23):4979. 10.3390/nu14234979
- Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF; STEP 1 Study Group (2021). Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med 384(11):989-1002. 10.1056/NEJMoa2032183
- Wilson-Pérez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D, Drucker DJ, Pérez-Tilve D, Seeley RJ (2013). Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes 62(7):2380-5. 10.2337/db12-1498
- Ye J, Hao Z, Mumphrey MB, Townsend RL, Patterson LM, Stylopoulos N, Münzberg H, Morrison CD, Drucker DJ, Berthoud HR (2014). GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol 306(5):R352-62. 10.1152/ajpregu.00491.2013
- Carpentier AC, Blondin DP, Haman F, Richard D (2023). Brown Adipose Tissue-A Translational Perspective. Endocr Rev 44(2):143-192. 10.1210/endrev/bnac015
- Hankir MK, Seyfried F (2020). Do Bariatric Surgeries Enhance Brown/Beige Adipose Tissue Thermogenesis? Front Endocrinol (Lausanne) 11:275. 10.3389/fendo.2020.00275
- Jin Z, Meng W, Xiao T, Deng J, Wang J, Wen J, Chen K, Wang L, Liu J, Li Q, He J, Wang Z, Liu W, Liu F (2023). Vertical sleeve gastrectomy-derived gut metabolite licoricidin activates beige fat thermogenesis to combat obesity. Theranostics 13(9):3103-3116. 10.7150/thno.81893
- Yadav J, Liang T, Qin T, Nathan N, Schwenger KJP, Pickel L, Xie L, Lei H, Winer DA, Maughan H, Robertson SJ, Woo M, Lou W, Banks K, Jackson T, Okrainec A, Hota SS, Poutanen SM, Sung HK, Allard JP, Philpott DJ, Gaisano HY (2023). Gut microbiome modified by bariatric surgery improves insulin sensitivity and correlates with increased brown fat activity and energy expenditure. Cell Rep Med 4(5):101051. 10.1016/j.xcrm.2023.101051
- Lutz TA, Bueter M (2016). The Use of Rat and Mouse Models in Bariatric Surgery Experiments. Front Nutr 3:25. 10.3389/fnut.2016.00025
- Stefanidis A, Lee CMC, Greaves E, Montgomery MK, Arnold M, Newn S, Budin AJ, Lemus MB, Foldi CJ, Burton PR, Brown WA, Lutz TA, Watt MJ, Oldfield BJ (2023). Mechanisms underlying the efficacy of a rodent model of vertical sleeve gastrectomy – A focus on energy expenditure. Mol Metab 73:101739. 10.1016/j.molmet.2023.101739
- Jahansouz C, Staley C, Kizy S, Xu H, Hertzel AV, Coryell J, Singroy S, Hamilton M, DuRand M, Bernlohr DA, Sadowsky MJ, Khoruts A, Ikramuddin S (2019). Antibiotic-induced Disruption of Intestinal Microbiota Contributes to Failure of Vertical Sleeve Gastrectomy. Ann Surg 269(6):1092-1100. 10.1097/SLA.0000000000002729
- Chung YW, Ahmad F, Tang Y, Hockman SC, Kee HJ, Berger K, Guirguis E, Choi YH, Schimel DM, Aponte AM, Park S, Degerman E, Manganiello VC (2017). White to beige conversion in PDE3B KO adipose tissue through activation of AMPK signaling and mitochondrial function. Sci Rep 7:40445. 10.1038/srep40445
- Seo DH, Shin E, Lee YH, Park SE, Nam KT, Kim JW, Cha BS (2022). Effects of a Phosphodiesterase inhibitor on the Browning of Adipose Tissue in Mice. Biomedicines 10(8):1852. 10.3390/biomedicines10081852
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH (2012). Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148(3):421-33. 10.1016/j.cell.2012.01.017
- Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, Wu J, Gunawardana SC, Banks AS, Camporez JP, Jurczak MJ, Kajimura S, Piston DW, Mathis D, Cinti S, Shulman GI, Seale P, Spiegelman BM (2014). Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156(1-2):304-16. 10.1016/j.cell.2013.12.021
- Li B, Li L, Li M, Lam SM, Wang G, Wu Y, Zhang H, Niu C, Zhang X, Liu X, Hambly C, Jin W, Shui G, Speakman JR (2019). Microbiota Depletion Impairs Thermogenesis of Brown Adipose Tissue and Browning of White Adipose Tissue. Cell Rep 26(10):2720-2737.e5. 10.1016/j.celrep.2019.02.015
- Wang D, Liu CD, Li HF, Tian ML, Pan JQ, Shu G, Jiang QY, Yin YL, Zhang L (2020). LSD1 mediates microbial metabolite butyrate-induced thermogenesis in brown and white adipose tissue. Metabolism 102:154011. 10.1016/j.metabol.2019.154011
- Simcox J, Geoghegan G, Maschek JA, Bensard CL, Pasquali M, Miao R, Lee S, Jiang L, Huck I, Kershaw EE, Donato AJ, Apte U, Longo N, Rutter J, Schreiber R, Zechner R, Cox J, Villanueva CJ (2017). Global Analysis of Plasma Lipids Identifies Liver-Derived Acylcarnitines as a Fuel Source for Brown Fat Thermogenesis. Cell Metab 26(3):509-522.e6. 10.1016/j.cmet.2017.08.006
- De Bandt JP, Coumoul X, Barouki R (2022). Branched-Chain Amino Acids and Insulin Resistance, from Protein Supply to Diet-Induced Obesity. Nutrients 15(1):68. 10.3390/nu15010068
- Bozadjieva Kramer N, Evers SS, Shin JH, Silverwood S, Wang Y, Burant CF, Sandoval DA, Seeley RJ (2020). The Role of Elevated Branched-Chain Amino Acids in the Effects of Vertical Sleeve Gastrectomy to Reduce Weight and Improve Glucose Regulation. Cell Rep 33(2):108239. 10.1016/j.celrep.2020.108239
- Chaudhari SN, Luo JN, Harris DA, Aliakbarian H, Yao L, Paik D, Subramaniam R, Adhikari AA, Vernon AH, Kiliç A, Weiss ST, Huh JR, Sheu EG, Devlin AS (2021). A microbial metabolite remodels the gut-liver axis following bariatric surgery. Cell Host Microbe 29(3):408-424.e7. 10.1016/j.chom.2020.12.004
- Chaudhari SN, Harris DA, Aliakbarian H, Luo JN, Henke MT, Subramaniam R, Vernon AH, Tavakkoli A, Sheu EG, Devlin AS (2021). Bariatric surgery reveals a gut-restricted TGR5 agonist with anti-diabetic effects. Nat Chem Biol 17(1):20-29. 10.1038/s41589-020-0604-z
–
ACKNOWLEDGMENTS
The author receives funding from the German Research Foundation (grant number HA 8213 3-1).
COPYRIGHT
© 2023

Gut microbiota turn up the heat after bariatric surgery by Mohammed K. Hankir is licensed under a Creative Commons Attribution 4.0 International License.



