News and thoughts:
Cell Stress, Vol. 7, No. 6, pp. 46 - 49; doi: 10.15698/cst2023.06.280
Novel insights at the crossroads of antibiotic use and cancer risk
1 Institute of Molecular Biosciences, University of Graz, NAWI Graz, Graz, Austria.
2 Field of Excellence BioHealth, University of Graz, 8010 Graz, Austria.
3 BioTechMed Graz, 8010 Graz, Austria.
4 Division of Biomedical Research, Core Facility Alternative Biomodels and Preclinical Imaging, Medical University of Graz, Graz, Austria.
Keywords: antibitiotics, cancer, immune cells, bacterial signature, antibacterial activity, anticancer activity, microbiome, drug resistance.
Received originally: 16/05/2023 Accepted: 23/05/2023
Published: 25/05/2023
Correspondence:
Nermina Malanovic, Institute of Molecular Biosciences, University of Graz, NAWI Graz, Graz, Austria; nermina.malanovic@uni-graz.at
Conflict of interest statement: Authors declare that there is no conflict of interest.
Please cite this article as: Nermina Malanovic and Djenana Vejzovic (2023). Novel insights at the crossroads of antibiotic use and cancer risk. Cell Stress 7(6): 46-49. doi: 10.15698/cst2023.06.280
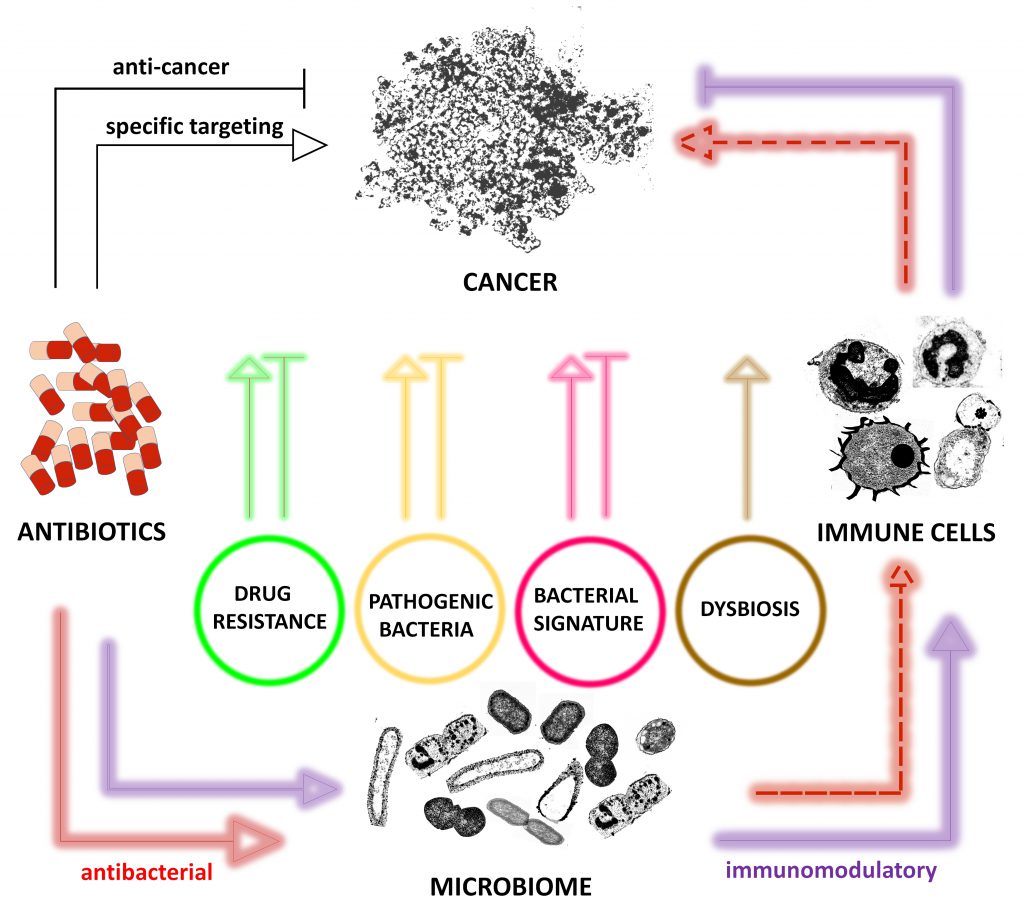
The continuous use of antibiotics is associated with the spread of antimicrobial resistances and the not yet clear link to cancer development. Many conventional antibiotics have already shown different effects on a variety of cancer types raising questions for their rational use in cancer. However, discrepancy in the observed trend for some antibiotics reducing cancer development and being associated with higher risk of cancer underscores the lack of understanding the complex link between antibiotics and cancer. Here, we briefly summarize the possible antibiotic-mediated effects on cancer and conclude that those effects can be direct via i) specific targeting of tumor/cancer, ii) antimicrobial activity and iii) immunomodulatory activity whereby iv) indirectly caused effects primarily affect immune equilibrium between bacteria, cancer and immune cells. Furthermore, we also conclude that there is a great need for bulk profiling, comprehensive screening programs in all countries and in-depth studies to understand the risks and benefits of antibiotic use.
Fleming’s discovery of the first natural antibiotic Penicillin in the late 1920’s [1] opened a golden era for modern medicine enabling the control of bacterial infections [2]. In the 21st century, however, their continuous use is associated with critical consequences that throw shadow on their success. These include the spread of antimicrobial resistances [3] and the not yet clear link to cancer development, as reports provide evidence for decreased survival of cancer patients exposed to antibiotics [4][5]. Global action plans and stewardship programs to fight antimicrobial resistance led the global antibiotic market to decline in 2021 from $21 to $8 billion for on-patent antibiotics, however, this is obviously not resulting in lower antibiotic use as the global antibiotic market continues to shift towards lower-cost generics that may raise further serious concerns about inappropriate use [6].
–
Recent articles report a strong association of malignant tumors with fungi and bacteria, which exhibit high immuno-suppressive properties and strongly promote cancer progression [7][8][9]. Various cancer types appear to be associated with a unique microbiome residing inside the cancer with immune cells actively disturbing the immune equilibrium [7][9][10][11]. Interestingly, decreased risk of rectal cancer was associated with past antibiotic use, most likely due to the anti-inflammatory impact of the used antibiotic tetracycline on a rectal inflammation associated with its oncogenesis [12][13][14]. Furthermore, researchers also point to growing evidence that an increased cancer risk arises more likely from an altered microbiome than from an inflammation in the gut. Also, treatment of mice bearing a colon cancer xenograft with the antibiotic metronidazole reduced the load of a cancer-colonized Fusobacterium, cancer cell proliferation, and overall tumor growth [15] questioning the innocent role of microbiota in cancer.
–
On the other hand, discrepancy in the observed trend for tetracyclines and macrolides reducing cancer development [16][17] and being associated with higher risk of (breast) cancer [17][18] underscores the lack of understanding the complex link between antibiotics and cancer. A recent study described the mechanism behind macrolide-induced tumor progression on melanoma and sarcoma in vitro and in mouse models in vivo, whereby macrolides block autophagic flux by inhibiting lysosomal acidification, inducing accumulation of radioactive species (ROS) and integrated stress response promoting tumor proliferation [19]. In patients, both breast and proximal colon cancer were linked to the use of other antibiotics including those targeting cell wall synthesis like penicillin or cephalosporins, but also to quinolones, sulfonamide and trimethoprim interfering with DNA and folate metabolism [12][13][20].
–
Apparently, there is no consensus, which antibiotic classes seem to be more associated with cancer prevalence. A summary by Gao and colleagues, discusses this double-edge sword of antibiotics used for cancer treatment and provides a rational basis for discussion of antibiotic use based on their modes of action [21]. Antibiotics in clinical use for bacterial infections can be classified according to the molecular mechanisms they use. A large majority of antibiotics target protein (tetracyclines, amphenicols, macrolides, aminoglycosides) and cell wall synthesis (glycopeptides, carbapenems, lipopeptides, phosphonates, cycloserines), but there are also antibiotics targeting DNA (azoles, fluoroquinolones), RNA (ansamycin, aipiarmycin) or their precursors through ATP or folate synthesis (sulfonamides) [22]. A common denominator for all those antibiotics is that although they inhibit a bacterial cell’s elemental processes essential for life, they unfortunately do not eliminate the bacteria from the host body. Hence, the distinct microbial signature (RNA, DNA, proteins, endotoxins etc.) remains in the body of the host which may further interfere with cellular processes in the host, particularly inducing inflammation that may result in multi organ failure and sepsis/septic shock [23][24][25][26]. Although the release of bacterial endo(toxins) depends on antibiotic used, the trend for rapid clinical deterioration increases [25]. In addition, it is also believed that bacteria by producing diverse proteins and enzymes prevent immune cells from killing cancer and prevent cancer cells from drug treatment and hence, are responsible for drug resistance [9][10]. In this context, it was shown in mice that tumors develop resistance to the chemotherapeutic drug gemcitabine in the presence of bacteria, which was reversed by antibiotic treatment [10]. Authors also point to the potential of antibiotic adjunctive treatment to cancer therapy.
–
Many conventional antibiotics have already shown different effects (Figure 1) on a variety of cancer types raising questions for their rational use in cancer [14][17][27][28]. However, the question arises whether antibiotics should be reconsidered as anticancerogenic, too. Indeed, some antibiotics showed anticancer effects independent of their antibacterial effects. Examples include flavoxin antibiotics which intake was beneficial for patients with lung and bladder cancer and flavoxin antibiotics were identified to inhibit p90 ribosomal protein S6 kinase 4 (RSK4), a common regulator of chemosensitivity in bladder and lung cancer [29].
–Although the link between antibiotics and cancer is growing, in discussion with renowned scientists, there is clear evidence that there is a great need for bulk profiling, comprehensive screening programs in all countries and in-depth studies to understand the risks and benefits of antibiotic use [13].
–
REFERENCES
- Fleming A (2001). On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. 1929. Bull WHO 79(8): 780–790. Available at: https://apps.who.int/iris/handle/10665/268396. [Accessed 15.05.2023]
- Cook MA, Wright GD (2022). The past, present, and future of antibiotics. Sci Transl Med 14(657): eabo7793. 10.1126/scitranslmed.abo7793
- Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, Han C, Bisignano C, Rao P, Wool E, Johnson SC, Browne AJ, Chipeta MG, Fell F, Hackett S, Haines-Woodhouse G, Kashef Hamadani BH, Kumaran EAP, McManigal B, Agarwal R, Akech S, Albertson S, Amuasi J, Andrews J, Aravkin A, Ashley E, Bailey F, Baker S, Basnyat B, Bekker A, et al. (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet 399(10325): 629–655. 10.1016/s0140-6736(21)02724-0
- Jing Y, Chen X, Li K, Liu Y, Zhang Z, Chen Y, Liu Y, Wang Y, Lin SH, Diao L, Wang J, Lou Y, Johnson DB, Chen X, Liu H, Han L (2022). Association of antibiotic treatment with immune-related adverse events in patients with cancer receiving immunotherapy. J Immunoth Cancer 10(1): e003779. 10.1136/jitc-2021-003779
- Ransohoff JD, Ritter V, Purington N, Andrade K, Han S, Liu M, Liang S-Y, John EM, Gomez SL, Telli ML, Schapira L, Itakura H, Sledge GW, Bhatt AS, Kurian AW (2023). Antimicrobial exposure is associated with decreased survival in triple-negative breast cancer. Nat Commun 14(1): 2053. 10.1038/s41467-023-37636-0
- Madden J, Outterson K (2023). Trends in the global antibiotics market. Nat Rev Drug Discov 22(3): 174. 10.1038/d41573-023-00029-5
- Galeano Niño JL, Wu H, LaCourse KD, Kempchinsky AG, Baryiames A, Barber B, Futran N, Houlton J, Sather C, Sicinska E, Taylor A, Minot SS, Johnston CD, Bullman S (2022). Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature 611(7937): 810–817. 10.1038/s41586-022-05435-0
- Narunsky-Haziza L, Sepich-Poore GD, Livyatan I, Asraf O, Martino C, Nejman D, Gavert N, Stajich JE, Amit G, González A, Wandro S, Perry G, Ariel R, Meltser A, Shaffer JP, Zhu Q, Balint-Lahat N, Barshack I, Dadiani M, Gal-Yam EN, Patel SP, Bashan A, Swafford AD, Pilpel Y, Knight R, Straussman R (2022). Pan-cancer analyses reveal cancer-type-specific fungal ecologies and bacteriome interactions. Cell 185(20): 3789-3806.e3717. 10.1016/j.cell.2022.09.005
- Sinha G (2022). Tumors can teem with microbes. But what are they doing there? Science 378(6621): 693–694. 10.1126/science.adf8359
- Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee K, Thaiss CA, Reuben A, Livny J, Avraham R, Frederick DT, Ligorio M, Chatman K, Johnston SE, Mosher CM, Brandis A, Fuks G, Gurbatri C, Gopalakrishnan V, Kim M, Hurd MW, Katz M, Fleming J, Maitra A, Smith DA, Skalak M, et al. (2017). Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357(6356): 1156–1160. 10.1126/science.aah5043
- Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E, Meltser A, Douglas GM, Kamer I, Gopalakrishnan V, Dadosh T, Levin-Zaidman S, Avnet S, Atlan T, Cooper ZA, Arora R, Cogdill AP, Khan MAW, Ologun G, Bussi Y, Weinberger A, Lotan-Pompan M, Golani O, Perry G, Rokah M, Bahar-Shany K, et al. (2020). The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368(6494): 973–980. 10.1126/science.aay9189
- Lu SSM, Mohammed Z, Häggström C, Myte R, Lindquist E, Gylfe Å, van Guelpen B, Harlid S (2022). Antibiotics Use and Subsequent Risk of Colorectal Cancer: A Swedish Nationwide Population-Based Study. J Natl Cancer Inst 114(1): 38–46. 10.1093/jnci/djab125
- Nelson B, Wiles A (2022). A growing link between antibiotics and colon cancer: Independent studies point to an association between antibiotic usage and proximal colon cancer, but a potential mechanism remains elusive: Independent studies point to an association between antibiotic usage and proximal colon cancer, but a potential mechanism remains elusive. Cancer Cytopathol 130(5): 318–319. 10.1002/cncy.22582
- Zhang J, Haines C, Watson AJM, Hart AR, Platt MJ, Pardoll DM, Cosgrove SE, Gebo KA, Sears CL (2019). Oral antibiotic use and risk of colorectal cancer in the United Kingdom, 1989-2012: a matched case-control study. Gut 68(11): 1971–1978. 10.1136/gutjnl-2019-318593
- Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, Neuberg D, Huang K, Guevara F, Nelson T, Chipashvili O, Hagan T, Walker M, Ramachandran A, Diosdado B, Serna G, Mulet N, Landolfi S, Ramon Y Cajal S, Fasani R, Aguirre AJ, Ng K, Élez E, Ogino S, Tabernero J, Fuchs CS, Hahn WC, Nuciforo P, Meyerson M (2017). Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358(6369): 1443–1448. 10.1126/science.aal5240
- Roderburg C, Loosen SH, Joerdens MS, Demir M, Luedde T, Kostev K (2023). Antibiotic therapy is associated with an increased incidence of cancer. J Cancer Res Clin Oncol 149(3): 1285–1293. 10.1007/s00432-022-03998-z
- Simin J, Tamimi RM, Engstrand L, Callens S, Brusselaers N (2020). Antibiotic use and the risk of breast cancer: A systematic review and dose-response meta-analysis. Pharmacol Res 160(105072. 10.1016/j.phrs.2020.105072
- Sørensen HT, Skriver MV, Friis S, McLaughlin JK, Blot WJ, Baron JA (2005). Use of antibiotics and risk of breast cancer: a population-based case-control study. Br J Cancer 92(3): 594–596. 10.1038/sj.bjc.6602313
- Yu X, Tian A-L, Wang P, Li J, Wu J, Li B, Liu Z, Liu S, Gao Z, Sun S, Sun S, Tu Y, Wu Q (2023). Macrolide antibiotics activate the integrated stress response and promote tumor proliferation. Cell Stress 7(4): 20–33. 10.15698/cst2023.04.278
- Zhang X, Yu L, Shi J, Li S, Yang S, Gao W, Yang S, Cheng M, Wang H, Guo Z, Geng C (2021). Antibiotics modulate neoadjuvant therapy efficiency in patients with breast cancer: a pilot analysis. Sci Rep 11(1): 14024. 10.1038/s41598-021-93428-w
- Gao Y, Shang Q, Li W, Guo W, Stojadinovic A, Mannion C, Man Y-G, Chen T (2020). Antibiotics for cancer treatment: A double-edged sword. J Cancer 11(17): 5135–5149. 10.7150/jca.47470
- Hutchings MI, Truman AW, Wilkinson B (2019). Antibiotics: past, present and future. Curr Opin Microbiol 51: 72–80. 10.1016/j.mib.2019.10.008
- Holzheimer RG (2001). Antibiotic induced endotoxin release and clinical sepsis: a review. J Chemother 13 Spec No 1(1): 159–172. 10.1179/joc.2001.13.Supplement-2.159
- Kellum JA, Foster D, Walker PM (2023). Endotoxemic Shock: A Molecular Phenotype in Sepsis. Nephron 147(1): 17–20. 10.1159/000525548
- Lepper PM, Held TK, Schneider EM, Bölke E, Gerlach H, Trautmann M (2002). Clinical implications of antibiotic-induced endotoxin release in septic shock. Intensive Care Med 28(7): 824–833. 10.1007/s00134-002-1330-6
- Poore GD, Kopylova E, Zhu Q, Carpenter C, Fraraccio S, Wandro S, Kosciolek T, Janssen S, Metcalf J, Song SJ, Kanbar J, Miller-Montgomery S, Heaton R, Mckay R, Patel SP, Swafford AD, Knight R (2020). Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 579(7800): 567–574. 10.1038/s41586-020-2095-1
- Armstrong D, Dregan A, Ashworth M, White P, McGee C, Lusignan Sd (2020). The association between colorectal cancer and prior antibiotic prescriptions: case control study. Br J Cancer 122(6): 912–917. 10.1038/s41416-019-0701-5
- Ford AC, Yuan Y, Moayyedi P (2020). Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut 69(12): 2113–2121. 10.1136/gutjnl-2020-320839
- Kingwell K (2021). Antibiotics switch to anticancer target. Nature Rev Drug Discov 20(9): 666. 10.1038/d41573-021-00138-z
–
COPYRIGHT
© 2023

Novel insights at the crossroads of antibiotic use and cancer risk by Malanovic and Vejzovic is licensed under a Creative Commons Attribution 4.0 International License.